| Identification | Back Directory | [Name]
Somatostatin | [CAS]
51110-01-1 | [Synonyms]
SRIF
GH-RIF
ay24910
panhibin
CCRIS 3629
Somatostatin
AGCKNFFWKTFTSC
SOMATOSTATIN-14
SOMATOSTATIN [GIF]
SOMATOSTATIN, SHEEP
SOMATOSTATIN [TYR1]
SOMATOSTATIN ACETATE
SOMATOSTATIN, CYCLIC
SOMATOSTATIN [D-TRP8]
Somatostatin USP/EP/BP
SOMATOSTATIN-14 (REDUCED)
M.W. 1637.80 C76H104N18O19S2
SOMATOSTATIN-14 (COUPLED TO BSA)
SOMATOSTATIN-14 (COUPLED TO KLH)
SOMATOTROPIN RELEASE INHIBITING FACTOR
AGCKNFFWKTFTSC (DISULFIDE BRIDGE: 3-14)
GIF (HUMAN, OVINE, PORCINE, RAT, MOUSE)
GROWTH HORMONE RELEASE INHIBITING FACTOR
SRIF (HUMAN, OVINE, PORCINE, RAT, MOUSE)
SOMATOSTATIN (HUMAN, OVINE, PORCINE, RAT, MOUSE)
GIF (HUMAN, OVINE, PORCINE, RAT, MOUSE) 2ACOH 6H2O
SRIF (HUMAN, OVINE, PORCINE, RAT, MOUSE) 2ACOH 6H2O
ALA-GLY-CYS-LYS-ASN-PHE-PHE-TRP-LYS-PHE-THR-SER-CYS-OH
ALA-GLY-CYS-LYS-ASN-PHE-PHE-TRP-LYS-THR-PHE-THR-SER-CYS
Anti-Somatostatin, N-Terminal antibody produced in rabbit
SOMATOSTATIN (HUMAN, OVINE, PORCINE, RAT, MOUSE) 2ACOH 6H2O
H-ALA-GLY-CYS-LYS-ASN-PHE-PHE-TRP-LYS-THR-PHE-THR-SER-CYS-OH
ALA-GLY-CYS-LYS-ASN-PHE-PHE-TRP-LYS-THR-PHE-THR-SER-CYS 2ACOH 6H2O
SOMATOTROPIN RELEASE INHIBITING FACTOR (HUMAN, OVINE, PORCINE, RAT, MOUSE)
GROWTH HORMONE RELEASE INHIBITING FACTOR (HUMAN, OVINE, PORCINE, RAT, MOUSE)
H-ALA-GLY-CYS-LYS-ASN-PHE-PHE-TRP-LYS-THR-PHE-THR-SER-CYS-OH (COUPLED TO BSA)
H-ALA-GLY-CYS-LYS-ASN-PHE-PHE-TRP-LYS-THR-PHE-THR-SER-CYS-OH (COUPLED TO KLH)
H-ALA-GLY-CYS-LYS-ASN-PHE-PHE-TRP-LYS-THR-PHE-THR-SER-CYS-OH, CYS3,14, CYCLIC
H-ALA-GLY-CYS-LYS-ASN-PHE-PHE-TRP-LYS-THR-PHE-THR-SER-CYS-OH (DISULFIDE BRIDGE: 3-14)
SOMATOTROPIN RELEASE INHIBITING FACTOR (HUMAN, OVINE, PORCINE, RAT, MOUSE) 2ACOH 6H2O
GROWTH HORMONE RELEASE INHIBITING FACTOR (HUMAN, OVINE, PORCINE, RAT, MOUSE) 2ACOH 6H2O
19,34-bis(4-aminobutyl)-31-(2-amino-2-oxoethyl)-37-[[2-(2-aminopropanoylamino)acetyl]amino]-13,25,28-tribenzyl-10,16-bis(1-hydroxyethyl)-7-(hydroxymethyl)-22-(1~{H}-indol-3-ylmethyl)-6,9,12,15,18,21,24,27,30,33,36-undecaoxo-1,2-dithia-5,8,11,14,17,20,23,26,29,32,35-undecazacyclooctatriacontane-4-carboxylic acid | [EINECS(EC#)]
256-969-7 | [Molecular Formula]
C76H104N18O19S2 | [MDL Number]
MFCD00076762 | [MOL File]
51110-01-1.mol | [Molecular Weight]
1637.88 |
| Questions And Answer | Back Directory | [Discovery]
This is a tetradecapeptide exerting a growth hormone
(GH) release-inhibiting activity. SS also has a large variety
of neuromodulatory and gastrointestinal actions, mostly as
an inhibitory hormone. SS of 14 aa residues (SS-14) was first isolated from
ovine hypothalamic extracts by Roger Guillemin’s group
in 1973 and was originally named GH inhibiting factor. An N-terminally extended form of 28 aa residues (SS-28)
was isolated later from the porcine gut. SS cDNA that
encodes a precursor for SS-14 and SS-28, a product of
the SS1 gene (approved symbol SST), was first cloned
from the anglerfish pancreas in 1980. In this report,
another SS cDNA encoding a different precursor (formerly called SSII), a product of the SS3 gene, was also isolated. Subsequently, cDNAs and genes encoding SS
precursors have been identified in many animals by
molecular cloning and bioinformatic analyses, and so
far six paralogous genes (SS1, SS2, SS3, SS4, SS5, and
SS6) have been identified in vertebrates. Accordingly,
two SS-related peptides, cortistatin (CST)5 and
neuronostatin, have been identified. | [Structure]
SS-14 and SS-28 contain a disulfide bridge and have a
cyclic structure. CST, a product of the SS2/CST
gene, is 14–17 aa residues in length, depending on the
species, and contains the SS2 signature, a proline residue
at position 2. CST in placental mammals exhibits an
additional lysine residue at its C-terminal extremity. Neuronostatin is a 13-residue amidated peptide that is
flanked with the signal peptide of the SS1 precursor, and is an acyclic peptide. The aa sequence of SS-14 is fully conserved in vertebrates. SS-28 in mammals that contains the SS-14 moiety
at its C-terminal shares 40%–66% sequence identity with
its counterparts in fish. Mr 1638 (SS-14), 3149 (SS-28). Soluble in water, acid,
and methanol. Stable in solution at -80°C for more than
a year. Plasma half-life is <3min.
 | [Gene, mRNA, and precursor]
The human preproSS gene (SS1), SST, location 3q28,
consists of two exons. Mammalian SS-14 and SS-28 are
derived from preproSS1 of 116 aa residues by specific
proprotein convertases (PCs) through tissue-specific
posttranslational processing. In vertebrates, six SS genes
have been identified, and all these paralogs are present in
teleost fish while only SS1 and SS2/CST are present in tetrapods. Phylogenetic and comparative genomic analyses
showed that whole-genome duplications, local duplications, and gene losses contribute to the divergent evolution of SS genes. | [Clinical implications]
Somatostatinoma is a malignant tumor that arises
from transformed D cells in the pancreatic islets or
duodenum. Somatostatinomas are associated with malabsorption, diabetes mellitus, steatorrhea, and cholelithiasis. In gastroenteropancreatic tumors, high levels of SS
receptor expression have been found, and specifically
designed analogs are used for tumor imaging and radiotherapy. SS deficiency causes persistent Helicobacter pylori
infection in the patient with chronic gastritis. | [Receptors]
SS receptors belong to the family of seventransmembrane-domain GPCRs. There are five subtypes
(sst1–sst5), and all these receptors bind to SS and CST with
high affinity.7 The structural and functional characteristics of these receptors in humans, including signal transduction pathways, agonists, and antagonists.
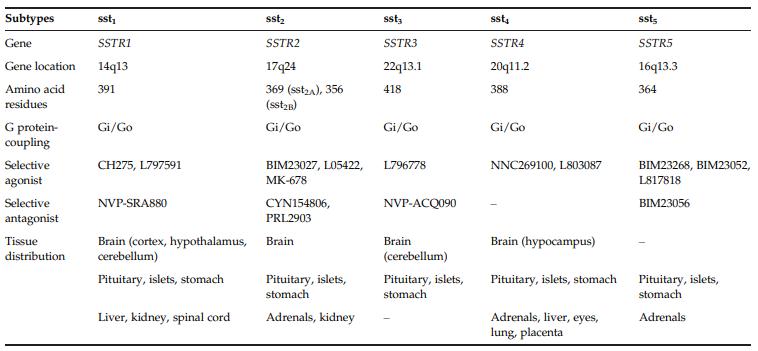 | [Biological functions]
In the anterior pituitary, SS inhibits the
release of GH and thyroid-stimulating hormone (TSH).
Pulsatile GH secretion reflects the pulsatile release of both
SS and GHRH in a reciprocal fashion. In fish, SS-14
inhibits the release of GH, prolactin, and insulin. In the
brain, SS has a variety of neuromodulatory roles in learning, cognitive functions, locomotor activity, anxiety, and
depression. CST has physiological functions, such as
depression of neuronal activity and induction of slowwave sleep. Moreover, SS exerts inhibitory effects on
various gastrointestinal functions, including gastric acid
secretion, gastric emptying, intestinal motility, and
release of insulin, glucagon, and various gastrointestinal
hormones. | [Regulation of synthesis and release]
SS secretion in the gastrointestinal tract is regulated by
the autonomous nervous system and various gut regulatory peptides including gastrin, cholecystokinin (CCK), and substance P. The synthesis and release of hypothalamic SS are regulated by GH, growth hormone-releasing
hormone (GHRH), and glucose. |
| Hazard Information | Back Directory | [Description]
Originally isolated from hypothalamic tissue, somatostatin is characterized as an inhibitor of growth hormone (GH) release.
The structure was determined in 1971. Subsequent investigations led to the recognition that somatostatin also was
released from the pancreas and has a role of inhibiting the secretion of both insulin and glucagon. A total of five
somatostatin receptor subtypes have been characterized and cloned (sst1 to sst5).
Subtype sst4 is associated with the
inhibition of insulin release, and an sst4-selective inhibitor has been reported. The somatostatin analogue SOM230
has exhibited selectivity for sst1, sst2, sst3, and sst5 in rats and effectively decreased plasma GH and insulin-like growth
factor-1 (IGF-1) levels by 75% without significant effects on insulin or glucagon. Another analogue, PT R3173, with
selectivity for recombinant human somatostatin receptor (hsst2, hsst4, hsst5) was substantially more effective in inhibiting
GH secretion compared to glucagon and insulin release in rats. | [Uses]
Somatostatin is a peptide hormone that regulates the endocrine system. | [Indications]
Somatostatin (51110-01-1) occurs primarily as a 14–amino acid peptide, although a 28–amino acid form also exists.As with the other hypothalamic peptides, it is formed by proteolytic cleavage of a larger precursor. Somatostatin, originally isolated from the hypothalamus, is also in many other locations, including the cerebral cortex, brainstem, spinal cord, gut, urinary system, and skin. Somatostatin inhibits the secretion of many substances in addition to growth hormone.
| [General Description]
Somatostatin was discovered in the hypothalamus. It is elaboratedby the δ-cells of the pancreas and elsewhere in thebody. Somatostatin is an oligopeptide (14 amino acidresidues) and is referred to as somatotropin release–inhibitingfactor (SRIF).
Its primary action is inhibiting the release of GH from thepituitary gland. Somatostatin also suppresses the release ofboth insulin and glucagon. It causes a decrease in bothcAMP levels and adenylate cyclase activity. It also inhibitscalcium ion influx into the pituitary cells and suppressesglucose-induced pancreatic insulin secretion by activatingand deactivating potassium ion and calcium ion permeability,respectively. The chemistry, SARs, and potential clinicalapplications have been reviewed. | [Clinical Use]
Somatostatin has a very brief half-life in serum and
is not useful clinically.An 8–amino acid analogue with 2
D-amino acids substituted for the naturally occurring
L-amino acids is more stable, and monthly injections of
a depot form of this analogue (octreotide, Sandostatin
LAR) have several uses. Long-acting octreotide is used
to treat acromegaly, as described earlier. It is also used
to counteract unpleasant effects caused by overproduction
of secreted bioactive substances produced by neuroendocrine
tumors, including hyperinsulinemia from
insulinomas and secretions from carcinoid tumors that
cause severe diarrhea. Octreotide may also control severe
diarrhea associated with AIDS that has not responded
to other treatments. | [Side effects]
Transient side effects, gastrointestinal discomfort
and decreased glucose tolerance, usually last only a few
weeks after initiation of therapy. The most significant
side effect associated with prolonged use of octreotide
is formation of gallstones resulting from reduced bile
flow. | [storage]
-20°C |
|
|