| Identification | Back Directory | [Name]
Curcumol | [CAS]
4871-97-0 | [Synonyms]
CURCUMOL
5,8-Epoxy-10(14)-guaien-8-ol
(3S,3aS,5S,6R,8aS)-Octahydro-3-methyl-8-methylene-5-(1-methylethyl)-6H-3a,6-epoxyazulen-6-ol
6H-3a,6-Epoxyazulen-6-ol, octahydro-3-methyl-8-methylene-5-(1-methylethyl)-, (3S,3aS,5S,6R,8aS)-
(3s-(3a,3aa,5a,6a,8ab))-octahydro-3-methyl-8-methylene-5-(1-methylethyl)-6h-3a,6-epoxyazulen-6-ol
(3S-(3alpha,3Aalpha,5alpha,6alpha,8abeta))-octahydro-3-methyl-8-methylene-5-(1-methylethyl)-6H-3A,6-epoxyazulen-6-ol | [Molecular Formula]
C15H24O2 | [MDL Number]
MFCD00272163 | [MOL File]
4871-97-0.mol | [Molecular Weight]
236.35 |
| Chemical Properties | Back Directory | [Melting point ]
141.0 to 145.0 °C | [Boiling point ]
334.5±42.0 °C(Predicted) | [density ]
1.06±0.1 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2–8 °C | [solubility ]
DMSO:55.67(Max Conc. mg/mL);235.54(Max Conc. mM)
DMF:20.0(Max Conc. mg/mL);84.62(Max Conc. mM)
Ethanol:28.5(Max Conc. mg/mL);120.58(Max Conc. mM) | [form ]
neat | [pka]
13.02±0.60(Predicted) | [color ]
White to Light yellow | [InChI]
InChI=1S/C15H24O2/c1-9(2)13-8-14-11(4)5-6-12(14)10(3)7-15(13,16)17-14/h9,11-13,16H,3,5-8H2,1-2,4H3/t11-,12-,13-,14-,15+/m0/s1 | [InChIKey]
QRMPRVXWPCLVNI-YYFQZIEXSA-N | [SMILES]
C1[C@]2([H])[C@@]3(O[C@](O)(CC2=C)[C@H](C(C)C)C3)[C@@H](C)C1 | [LogP]
3.276 (est) | [CAS DataBase Reference]
4871-97-0 |
| Questions And Answer | Back Directory | [Background]
Curcumol, a bioactive sesquiterpenoid, has been isolated from numerous plants of family Zingiberaceae. These plants are mostly found in Southeast Asia, China, Indonesia, India, Peru, and West Indies. Curcumol has been isolated from C. longa which is an imperative species of the genus Curcuma and is generally known as common turmeric. Curcumol was also extracted from the rhizome of C. aeruginosae that is effective for antiinflammatory and antioxidant activities. C. aromatic, a well-known natural source of curcumol, has been reported for its antitumor and antimicrobial activities. C. kwangsiensis and C. phaeocaulis are advantageous for their antiproliferative activities against various cancer types. Dried roots of C. zedoary enriched with curcumol are documented for their effective anticancer and antiinflammatory properties. Rhizome of various Curcuma species, a rich source of curcumol, serves as antimicrobial, antifibrotic, and anticancer agent. Besides them, curcumol has also been isolated from the roots of Astragali radix, C. rhizoma, and Fructus gardenia which are traditionally used as anticancer agents.
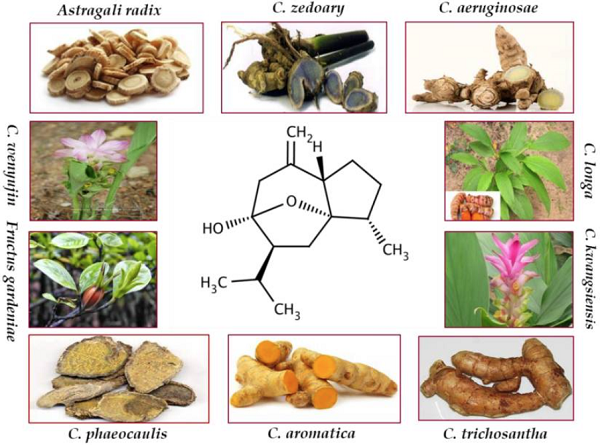
The above figure provides natural sources of curcumol including Astragali radix, C. aeruginosae, C. aromatica, C. kwangsiensis, C. longa, C. phaeocaulis, C. wenyujin, C. zedoary, Fructus gardenia, and C. trichosantha G[1].
|
| Hazard Information | Back Directory | [Description]
Curcumol, a bioactive sesquiterpenoid, has been isolated from various plants from Zingiberaceae. In recent years, curcumol attracts much attention due to its multiple biological activities and few negative effects. Accumulated studies prove that curcumol shows beneficial effects in protecting against inflammation, oxidative stress, cancer, neurodegeneration and microbial infection. Besides, a number of signaling pathways such as MAPK, PI3K/Akt and NF-κB are influenced by curcumol[2]. | [Uses]
Curcumol is a potential treatment for advanced hepatocellular carcinoma (HCC). It can be used as a chemotherapeutic sensitiser in order to enhance the anti-tumour effect of chemotherapeutic drugs. Studies have shown that it promotes HCC cell apoptosis in vitro and inhibits HCC tumour growth in vivo. It combined with lenvatinib can induce more autolysosome formation, which can enhance the anti-tumour effect of lenvatinib on hepatocellular carcinoma cells. | [Definition]
ChEBI: Curcumol is a sesquiterpenoid. | [Biological Activity]
Curcumol is a biologically active sesquiterpene with many pharmacological activities such as anticancer, antimicrobial, antifungal, antiviral and anti-inflammatory. It efficiently induces apoptosis in many cancer cells by targeting key signaling pathways such as MAPK/ERK, PI3K/Akt and NF-κB, which are often dysregulated in several cancers. | [Pharmacokinetics]
Curcumol is rapidly absorbed and eliminated in rats following oral administration of 10, 40 and 80 mg/kg curcumol. Its oral and intravenous bioavailability ranges from 9.2% ~ 13.1%. Maximum concentration is reached within 0.5-1.0 hours. In addition, high protein binding of curcumin was observed in rat plasma, ranging from 85.6% to 93.4%. | [in vitro]
Curcumol ((-)-Curcumol) is the major component extracted from root of Rhizoma Curcumae . Curcumol exerts anticancer effect in cholangiocarcinoma cells via down-regulating CDKL3. It induces cell cycle arrest in colon cancer cells via reactive oxygen species and Akt/ GSK3β/cyclin D1 pathway. It suppresses breast cancer cell metastasis by inhibiting MMP-9 via JNK1/2 and Akt-dependent NF-κB signaling pathways. | [storage]
Store at 4°C | [References]
[1] Wei Wei. “Curcumol: From Plant Roots to Cancer Roots.” International Journal of Biological Sciences 27 1 (2019): 1600–1609.
[2] Ying Liu. “Curcumol ameliorates neuroinflammation after cerebral ischemia-reperfusion injury via affecting microglial polarization and Treg/Th17 balance through Nrf2/HO-1 and NF-κB signaling.” Cell Death Discovery 10 1 (2024): 300.
|
|
|