| Identification | Back Directory | [Name]
N-METHYL-L-PROLINE MONOHYDRATE | [CAS]
475-11-6 | [Synonyms]
N-ME-PRO-OH
Hygric acid
N-ME-PROLINE
Hygrinic acid
N-Me-L-Pro-OH
H-MEPRO-OH H2O
PYRD(2)-OH H2O
N-ME-PRO-OH HCL
N-Methylproline
1-Methylproline
MEPYRD(2)-OH H2O
(-)-Hygrinic acid
1-Methyl-L-proline
N-METHYL-L-PROLINE
N-Methyl-L-proline98+%
N-ALPHA-METHYL-L-PROLINE
N-ME-L-PROLINE HYDROCHLORIDE
N-METHYL-L-PROLINE MONOHYDRATE
N-ALPHA-METHYL-L-PROLINE HYDRATE
N-METHYL-L-PROLINE MONOHYDRATE HCL
(2S)-1-Methyl-2-pyrrolidinecarboxylic acid
(2S)-1-Methylpyrrolidine-2-carboxylic acid
N-ALPHA-METHYL-L-PYRROLIDINE-2-CARBOXYLIC ACID HYDRATE
tert-butyl N-[4-(aminomethyl)cyclohexyl]carbamate,hydrochloride | [Molecular Formula]
C6H11NO2 | [MDL Number]
MFCD00149962 | [MOL File]
475-11-6.mol | [Molecular Weight]
129.16 |
| Chemical Properties | Back Directory | [Melting point ]
114-116 °C(lit.)
| [Boiling point ]
227℃ | [density ]
1.153 | [Fp ]
91.1℃ | [refractive index ]
1.5000 | [storage temp. ]
−20°C
| [solubility ]
Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | [form ]
Powder | [pka]
2.37±0.20(Predicted) | [color ]
White to off-white | [Water Solubility ]
Soluble in water and methanol(both 50 mg/ml-clear-colorless solution) | [InChI]
InChI=1S/C6H11NO2/c1-7-4-2-3-5(7)6(8)9/h5H,2-4H2,1H3,(H,8,9)/t5-/m0/s1 | [InChIKey]
CWLQUGTUXBXTLF-YFKPBYRVSA-N | [SMILES]
C(O)(=O)[C@@H]1CCCN1C | [CAS DataBase Reference]
475-11-6 |
| Hazard Information | Back Directory | [Definition]
ChEBI: An L-proline derivative obtained by replacement of the amino hydrogen by a methyl group. | [Uses]
N-Methyl-L-proline, is an aminoacid which is widely used in pharmaceuticals and food industry. | [Biological Activity]
N-Methyl-L-proline is a proline analogue present in citrus fruits. | [Synthesis]
L-Proline 86 (2.0 g, 17.4 mmol) was dissolved in methanol (20 mL) and to this solution was added 40% aqueous formaldehyde solution (1.4 mL, 19.1 mmol). This was followed by the addition of 10% palladiumon-charcoal catalyst (500 mg) and the resulting slurry was stirred in a hydrogen atmosphere overnight. The slurry was then filtered through a Celite pad to remove the catalyst. The pad was washed with methanol and the combined filtrates were concentrated under reduced pressure. The residue was taken up in ethanol-benzene (1:1, 100 mL) and concentrated a second time to provide a solid, which was recrystallized from methanol-diethyl ether. In this way N-Methyl-L-proline was isolated as fine needles (2.2 g, 98%). Mp 142-145 °C. [α]23 D -78.0° (c 2.0, MeOH). 1H NMR (300 MHz, D2O) δ 3.71-3.65 and 3.55-3.51 (2m, 1H), 3.00-2.91 (m, 1H), 2.74 (s, 3H), 2.34-2.28 (m, 1H), 1.99-1.78 (m, 3H). 13C NMR (75 MHz, CDCl3) δ 173.06, 70.18, 55.83, 40.26, 28.34, 22.37. IR (KBr disk) ν 3000-2800 (CH, saturated), 2675 and 2605 (ammonium ion), 1669 (CO2H), 1612 (CO2 -), 1468, 1401, 1354, 1327, 1234, 1183, 1112, 1056, 1025, 808, 775 cm-1. HRMS calcd for C6H11NO2 (M+) 129.0790, found 129.0784.
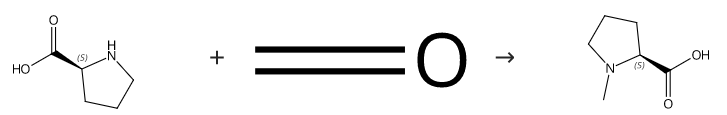 |
|
|