| Identification | Back Directory | [Name]
9-methylidenefluorene | [CAS]
4425-82-5 | [Synonyms]
9-methylidenefluorene
Fluorenylidenemethane
9-Methylene-9H-fluorene
9H-Fluorene, 9-Methylene- | [Molecular Formula]
C14H10 | [MDL Number]
MFCD11850122 | [MOL File]
4425-82-5.mol | [Molecular Weight]
178.23 |
| Chemical Properties | Back Directory | [Melting point ]
46-48 °C | [Boiling point ]
305.9±12.0 °C(Predicted) | [density ]
1.12±0.1 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly) | [form ]
Solid | [color ]
Off-White to Pale Beige | [Stability:]
Light Sensitive |
| Hazard Information | Back Directory | [Description]
9-Methylene-9H-fluorene is a fluorescent probe that can be used to detect the presence of hydroxyl groups in vitro. It reacts with the hydroxyl group on the substrate film and emits light, which is then detected using a fluorometer. The reaction mechanism is shown below. Fluorophores are often used for sample preparation because they are relatively stable and can be easily removed from the final product. 9-Methylene-9H-fluorene has been shown to enhance cationic polymerization, which can lead to improved efficacy in some applications. | [Chemical Properties]
9-methylidenefluorene or dibenzofulvene (DBF) is a polycyclic aromatic hydrocarbon with chemical formula C14H10.
9-methylidenefluorene is an intermediate of Fmoc cleavage reaction. It is an analog of a 1,1-diphenylethylene. Polymerization of 9-methylene-fluorene produces a π-stacked polymer. | [Uses]
9-Methylidenefluorene is an analog of 9-Methylfluorene (M305335); a compound that has been shown to be mutagenic in Salmonella typhimurium TA98 and TA100 in the presence of 9000 X g supernantant from Aroclor-induced rats. | [Application]
9-Methylene-fluorene is an intermediate of Fmoc cleavage reaction. It is an analog of a 1,1-diphenylethylene. Polymerization of 9-methylene-fluorene produces a π-stacked polymer. | [Preparation]
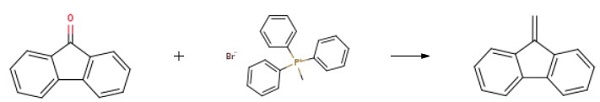
A suspension of methyl triphenylphosphonium bromide (31.22 g; 87.3 mmol) in 500 mL of ether was added at -30°C. 10M n-butyllithium in hexane (8.73 mL; 87.3 mmol) was added to the mixture, and the mixture was stirred at room temperature for 1-2 hours.
To the above mixture, 15 g (83.2 mmol) of 9-fluorenone in 50 mL of tetrahydrofuran was added, and the mixture was refluxed for 1 hour.
After cooling the above mixture to 0°C, 9-Methylene-9H-fluorene was obtained after purification.
 |
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|