| Identification | Back Directory | [Name]
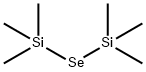
[BIS(TRIMETHYLSILYL)]SELENIDE | [CAS]
4099-46-1 | [Synonyms]
[BIS(TRIMETHYLSILYL)]SELENIDE
Bis(trimethylsilyl)selenide,98% | [Molecular Formula]
C6H18SeSi2 | [MDL Number]
MFCD00143781 | [MOL File]
4099-46-1.mol | [Molecular Weight]
225.34 |
| Chemical Properties | Back Directory | [Appearance]
CLEAR COLOURLESS TO YELLOW LIQUID | [Melting point ]
-7°C | [Boiling point ]
58-9°C/11mmHg | [density ]
0,9 g/cm3 | [refractive index ]
1.481 | [Fp ]
59.6±18.7℃ | [solubility ]
sol Et2O and THF. | [form ]
liquid | [Specific Gravity]
0.90 | [Hydrolytic Sensitivity]
7: reacts slowly with moisture/water |
| Hazard Information | Back Directory | [Chemical Properties]
CLEAR COLOURLESS TO YELLOW LIQUID | [Physical properties]
bp 45–46°C/5.3 mmHg. | [Uses]
Bis(trimethylsilyl) Selenide can be used as synthesis of unsymmetrical selenides; generation of selenoaldehydes;
reduction of sulfoxides, selenoxides, and
telluroxides).
Bis(trimethylsilyl)
selenide reacts with equimolar amounts of n-butyllithium to generate
Me3SiSeLi, alkylation of which then provides trimethylsilyl
alkyl selenides. Similar treatment of Me3SiSeR with BuLi /
R2X successfully leads to unsymmetrical selenides in good yields
(eq 5).Use of acid chlorides in place of alkyl halides results in
the formation of selenoesters. | [Preparation]
In the initial studies,
bis(trimethylsilyl) selenide was synthesized by the following
two methods: silylation of sodium selenide (or lithium selenide)
with chlorotrimethylsilane (eq 1) or reaction of bromobenzene,
magnesium, and selenium with chlorotrimethylsilane (eq 2).
|
|
|