| Identification | Back Directory | [Name]
5-(Trifluoromethyl)-1H-1,2,3-triazole | [CAS]
40964-54-3 | [Synonyms]
5-trifluoromethyl-1,2,3-triazole
4-(trifluoromethyl)-1H-1,2,3-triazole
5-(Trifluoromethyl)-1H-1,2,3-triazole
1H-1,2,3-Triazole, 4-(trifluoromethyl)-
1H-1,2,3-Triazole, 5-(trifluoromethyl)- | [Molecular Formula]
C3H2F3N3 | [MOL File]
40964-54-3.mol | [Molecular Weight]
137.06 |
| Hazard Information | Back Directory | [Definition]
5-(Trifluoromethyl)-1H-1,2,3-triazole is a fluorinated triazole derivative. Triazoles are an important class of organic compounds, playing an important role in modern medicinal chemistry. Compounds with 1,2,3- and 1,2,4-triazole moieties are known to possess fungicidal, antimicrobial, anticonvulsant, antidepressant, antibacterial, anti-inflammatory, analgesic and antitumor activities[1].
| [Synthesis]
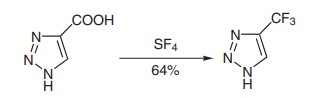
A mixture of 1H-1,2,3-Triazole-4-carboxylic Acid (39.0 g, 345 mmol), anhyd HF (50 mL) and SF4 (46.5 g, 430 mmol) in a 500-mL autoclave was heated at 120 °C (oil bath temperature) for 16 h. The reaction mixture was cooled to 0 °C and the autoclave was then opened. The residue was put on a Teflon plate; after the residual HF had been removed by heating at 30 °C for 1–2 h (CAUTION! This procedure must be carried out in a fume hood!), the product was dissolved in CH2Cl2 (300 mL) to remove all insoluble materials. The solution was evaporated under vacuum and the thus-formed tarry residue was purified by double sublimation (20 mmHg, ~80–90 °C) to give 5-(Trifluoromethyl)-1H-1,2,3-triazole (30.3 g, 221 mmol, 64%) as a white solid[1].
 | [References]
[1] Mykhailiuk P, et al. Novel Synthetic Approaches to (Trifluoromethyl)triazoles. Synthesis, 2010; 7: 1075-1077.
|
|
|