| Identification | Back Directory | [Name]
Azlocillin | [CAS]
37091-66-0 | [Synonyms]
Azlocillin
AZLOCILLIN ACID
Azlocillin USP/EP/BP
Azlocillin solution,100ppm
6α-[[(R)-[[(2-Oxo-1-imidazolidinyl)carbonyl]amino]phenylacetyl]amino]penicillanic acid
3,3-Dimethyl-6-oxo-7-[2-(2-oxoimidazolidin-1-yl)carbonylamino-2-phenyl-acetyl]amino-2-thia-5-azabicyclo[3.2.0]heptane-4-carboxylic acid
3,3-Dimethyl-7-oxo-6-[[[[(2-oxoimidazolidin-1-yl)carbonyl]amino]phenylacetyl]amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
(2S,5R,6R)-3,3-Dimethyl-7-oxo-6-((R)-2-(2-oxoimidazolidine-1-carboxamido)-2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
[2S-[2a,5a,6(s*)]]3,3-dimethyl-7-oxo-6{[[[[(2-oxoimideazolidine-1-yl)carbonyl]amino]phenylacethyl]-amino]-4-thia-1-azabicyclo[3,2,0]-heptane-2-carboxylic acid
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-[[(2R)-2-[[(2-oxo-1-imidazolidinyl)carbonyl]amino]-2-phenylacetyl]amino]-, (2S,5R,6R)-
(2S,5R,6R)-3,3-dimethyl-7-oxo-6-[[(2S)-1-oxo-2-[[oxo-(2-oxo-1-imidazolidinyl)methyl]amino]-2-phenylethyl]amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid | [EINECS(EC#)]
253-348-2 | [Molecular Formula]
C20H23N5O6S | [MDL Number]
MFCD00864967 | [MOL File]
37091-66-0.mol | [Molecular Weight]
461.492 |
| Chemical Properties | Back Directory | [Melting point ]
157 - 160oC | [density ]
1.55±0.1 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
pKa 2.8 (Uncertain) | [color ]
White to Off-White |
| Hazard Information | Back Directory | [Originator]
Securopen,Bayer,W. Germany,1977 | [Uses]
Antibacterial. | [Definition]
ChEBI: A semisynthetic penicillin antibiotic used in treating infections caused by Pseudomonas aeruginosa, Escherichia coli, and Haemophilus influenzae. | [Indications]
Azlocillin is active with respect to Gram-positive and Gram-negative aerobic and anaero�bic microorganisms. It is highly effective with respect to bacillus pyocyaneus, including
strains that are resistant to carbenicillin and aminoglycosides. It is destroyed by beta�lactamases. It is used for bacterial infections such as pyelonephritis, uretritis, cystitis,
endometritis, cholecystitis, sepsis, peritonitis, endocarditis, meningitis, pneumonia, infec�tions of the skin and soft tissues, infected burns, and so on. Synonyms of this drug are
securopen and azlin. | [Manufacturing Process]
3.8 parts by weight of D-(-)-α-[(imidazolidin-2-on-1-yl)carbonylamino]phenylacetic
acid were dissolved in 65 parts by volume of dichloromethane. 2.7
parts by weight of 1-methyl-2-chloro-δ1-pyrrolinium chloride were added, and
after cooling to -10°C 2.0 parts by volume of triethylamine were added
gradually. This reaction mixture was then stirred for one hour at -5°C
(mixture A). 4.0 parts by weight of 6-aminopenicillanic acid in 80 parts by
volume of dichloromethane were treated with 4.4 parts by volume of
triethylamine and 4.0 parts by weight of anhydrous sodium sulfate and then
stirred for two hours at room temperature. After filtration, the solution was
cooled to -20°C and combined with the mixture A. The reaction mixture was
left to reach 0°C of its own accord, and was then stirred for a further hour at
0°C. The solvent was removed in a rotary evaporator, the residue was
dissolved in water, and the solution was covered with a layer of ethyl acetate and acidified with dilute hydrochloric acid at 0° to 5°C, while stirring, until pH
1.5 was reached. The organic phase was then separated off, washed with
water, dried over magnesium sulfate while cooling, and filtered, and after
dilution with an equal amount of ether the sodium salt of the penicillin was
precipitated from the filtrate by adding a solution of sodium 2-ethylcaproate
dissolved in ether containing methanol. Yield: 1.3 parts by weight. | [Brand name]
Azlin (Bayer). | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
A semisynthetic acylureidopenicillin supplied as the sodium
salt for parenteral administration. It is active against a wide
range of other Gram-negative bacteria, but is distinguished
mainly by its activity against Ps. aeruginosa. B.
fragilis and other anaerobes are moderately susceptible. Like
other ureidopenicillins, azlocillin is active against Grampositive
cocci, H. influenzae and N. gonorrhoeae. Because it can
be hydrolyzed by most β-lactamases, β-lactamase-producing
isolates are resistant.
It attains peak concentrations of 250 mg/L after a 3 g intravenous
infusion, with a plasma half-life of approximately 1 h.
Protein binding is 20–30%. It distributes into multiple tissues
and human body fluids at therapeutically useful concentrations.
Up to 60% of the dose is recoverable from the urine,
mostly unchanged, although some hydrolysis of the β-lactam
ring takes place in the body.
Toxicity and side effects are similar to those associated
with carboxypenicillins. Its clinical use is for serious infections
with susceptible organisms, including lower respiratory tract,
intra-abdominal, urinary tract and gynecological infections.
Commercial availability is quite limited. | [Synthesis]
Azlocillin, (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(R)-2-(2-oxoimidazolidin-1-carbox�amido)-2-phenylacetamido]-4-thia-1-azabicyclo[3.2.0]-heptan-2-carboxylic acid (32.1.1.24),
is synthesized by the following scheme. 2-Imidazolidinone is acylated with phosgene, forming
1-chlorocarbonyl-2-imidazolidinone (32.1.1.22). The resulting 1-chlorocarbonyl-2-imidazo�lidinone (32.1.1.22) is reacted with D(?)-|á-phenylglycine, forming N-(2-oxoimidazolidin-
1-carboxamido)-phenylglycine (32.1.1.23). Reacting this with 6-APA in the presence of tri�ethylamine gives the desired azlocillin (32.1.1.24).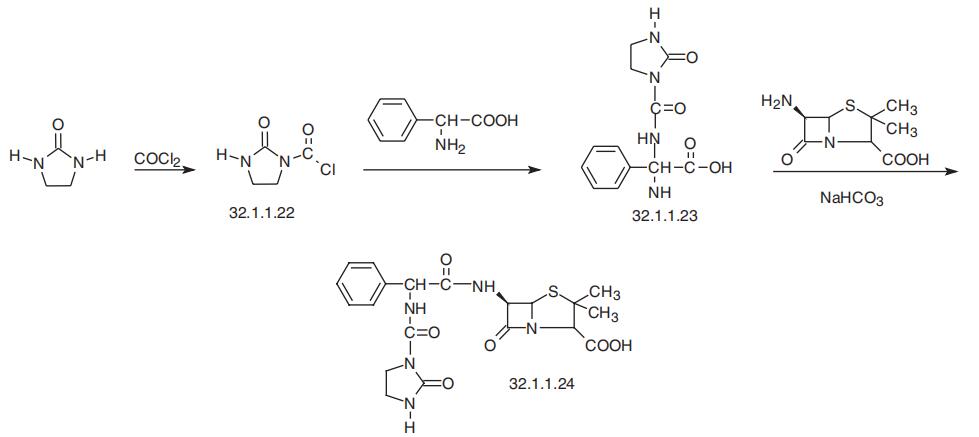
|
|
|