| Identification | Back Directory | [Name]
1-BUTYL-METHYLPYRROLIDINIUM TRIFLUOROMETHANESULFONATE | [CAS]
367522-96-1 | [Synonyms]
SKL1447
SKL1615
PP1,4OTF
BMPyrr OTf
[C4MPr]TfS
1-Butyl-1-methylpyrrolidinium Triflate
1-BUTYL-METHYLPYRROLIDINIUM TRIFLUOROMETHANE
N-butyl,methylpyrrolidinium trifluoroacetate
N-butyl-N-MethylpyrrolidiniuM trifluoroacetate
1-BUTYL-METHYLPYRROLIDINIUM TRIFLUOROMETHANESULFONATE
1-Butyl-1-methylpyrrolidin-1-ium 2,2,2-trifluoroacetate
N-Butyl-N-methylpyrrolidinium trifluoromethanesulfonate
1-butyl-1-methylpyrrolidin-1-ium:trifluoromethanesulfonate
1-Butyl-1-Methylpyrrolidinium Trifluoromethanesulfonate,>98%
1-Butyl-1-methylpyrrolidinium trifluoromethanesulphonate, 95% | [Molecular Formula]
C10H20F3NO3S | [MDL Number]
MFCD09038885 | [MOL File]
367522-96-1.mol | [Molecular Weight]
291.331 |
| Chemical Properties | Back Directory | [Melting point ]
4°C(lit.) | [density ]
1.25 g/cm3 (20℃) | [refractive index ]
1.4320 to 1.4360 | [storage temp. ]
Store below +30°C. | [form ]
clear liquid | [color ]
Colorless to Light yellow to Light orange | [PH]
6 (H2O, 25℃) | [BRN ]
9820503 | [InChI]
InChI=1S/C9H20N.CHF3O3S/c1-3-4-7-10(2)8-5-6-9-10;2-1(3,4)8(5,6)7/h3-9H2,1-2H3;(H,5,6,7)/q+1;/p-1 | [InChIKey]
WZJDNKTZWIOOJE-UHFFFAOYSA-M | [SMILES]
S(=O)(=O)([O-])C(F)(F)F.[N+]1(CCCC1)(C)CCCC | [ECW]
4.2 V |
| Hazard Information | Back Directory | [Conductivity]
1.85 mS/cm (24 °C) | [Uses]
1-Butyl-1-methylpyrrolidinium trifluoromethanesulfonate ([BMPy][OTf]) is an ionic liquid that can be used as a solvent in:
- Rhodium-catalyzed regioselective hydroformylation reactions.
- Direct asymmetric aldol condensation reaction.
- Desulfurization of fuels.
- Nucleophilic aromatic substitution reactions.
([BMPy][OTf]) can also be used as an electrolyte in supercapacitor applications. | [Preparation]
First, the N, N'-butylmethylpyridinium chloride and potassium trifluoromethanesulfonate are mixed in acetone and reacted for 24 h. The reaction solution is filtered to remove solid impurities. Finally, acetone in the solution is removed by distillation and purified to obtain 1-Butyl-1-methylpyrrolidinium trifluoromethanesulfonate.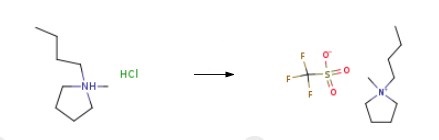
|
|
|