| Identification | Back Directory | [Name]
H-HIS(TRT)-OH | [CAS]
35146-32-8 | [Synonyms]
His(Trt)
L-His(Trt)
H-HIS(TRT)-OH
L-His(Trt)-OH
L-His(Trt)·H2O
H-L-HIS(TRT)-OH
H-HIS(1-TRT)-OH
H-HIS(T-TRT)-OH
H-His(tau-Trt)-OH
RARECHEM AL BE 1480
HISTIDINE(1-TRT)-OH
N-IM-TRITYL-HISTIDINE
N-τ-Trityl-L-histidine
N-IM-TRITYL-L-HISTIDINE
H-HIS(TRT)-OH USP/EP/BP
N-TAU-TRITYL-L-HISTIDINE
1-Trityl-L-histidine, 98%
N-TRITYL-L-HISTIDINE
1-(Triphenylmethyl)-L-histidine
Nim-Trityl-histidine≥ 98% (HPLC)
L-Histidine, 1-(triphenylmethyl)-
(2S)-2-amino-3-(1-tritylimidazol-4-yl)propanoicaci
(2S)-2-amino-3-(1-tritylimidazol-4-yl)propanoicacid
(2S)-2-ammonio-3-(1-trityl-1H-imidazol-4-yl)propanoate
(S)-2-Amino-3-(1-trityl-1H-imidazol-4-yl)propanoicacid
(2S)-2-amino-3-[1-(triphenylmethyl)-4-imidazolyl]propanoic acid
(2S)-2-amino-3-[1-(triphenylmethyl)-1H-imidazol-4-yl]propanoic acid | [EINECS(EC#)]
609-078-6 | [Molecular Formula]
C25H23N3O2 | [MDL Number]
MFCD00153444 | [MOL File]
35146-32-8.mol | [Molecular Weight]
397.47 |
| Chemical Properties | Back Directory | [Melting point ]
210℃ | [Boiling point ]
584.8±50.0 °C(Predicted) | [density ]
1.19±0.1 g/cm3(Predicted) | [storage temp. ]
−20°C
| [form ]
Solid | [pka]
1.78±0.10(Predicted) | [color ]
White to off-white | [InChI]
InChI=1S/C25H23N3O2/c26-23(24(29)30)16-22-17-28(18-27-22)25(19-10-4-1-5-11-19,20-12-6-2-7-13-20)21-14-8-3-9-15-21/h1-15,17-18,23H,16,26H2,(H,29,30)/t23-/m0/s1 | [InChIKey]
BSZQZNOAYQCQFZ-QHCPKHFHSA-N | [SMILES]
C(O)(=O)[C@H](CC1N=CN(C(C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)C=1)N |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Uses]
1-(Triphenylmethyl)-L-histidine is a reactant in the synthesis of falcitidin acyl tetrapeptides as antimalarial agent. | [Definition]
H-His(Trt)-OH, also known as 1-(Triphenylmethyl)-L-histidine, is a histidine derivative. It can be obtained by removing fmoc group protection from Fmoc-His(Trt)-OH. L-histidine (HIS) is an essential amino acid with unique roles in proton buffering, metal ion chelation, scavenging of reactive oxygen and nitrogen species, erythropoiesis, and the histaminergic system. | [Preparation]
The amino acid N(im)-trityl-D-histidine was dissolved in dichloromethane, followed by the addition of triethylamine and trimethylsilyl chloride (3.5 equivalents). After 2 hours of gentle reflux, the mixture was ready to be used for in situ coupling to the anhydride of the carboxylic acid.
Thus, isovaleric acid was separately dissolved in THF and maintained at -20 ℃ using an ice-salt bath. N-methyl morpholine (1.2 equivalents) was added, followed by the addition of ethyl chloroformate (1.0 equivalent). After 15-20 minutes at -20 ℃, the bis-TMS amino acid reaction mixture was added directly to the anhydride solution and left stirring at room temperature overnight. After 8 hours, the reaction mixture was concentrated in vacuo.
Finally, H-His(Trt)-OH was obtained after purification.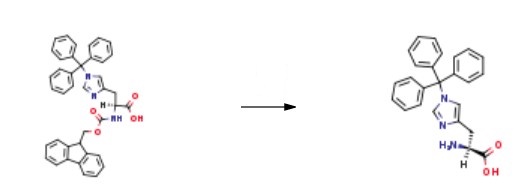
|
|
|