| Identification | Back Directory | [Name]
L-THREONINE BENZYL ESTER HYDROCHLORIDE | [CAS]
33645-24-8 | [Synonyms]
L-Thr-OBzl HCl
L-THREONINE BENZYL ESTER HCL
Benzyl L-threoninate hydrochloride
L-THREONINE BENZYL ESTER HYDROCHLORIDE
L-THREONINE BENZYL ESTER HYDROCHLORIDE USP/EP/BP
L-Threonine Benzyl Ester Hydrochloride
benzyl (2S,3R)-2-amino-3-hydroxybutanoate hydrochloride
(2S,3R)-Benzyl 2-aMino-3-hydroxybutanoate hydrochloride | [Molecular Formula]
C11H16ClNO3 | [MDL Number]
MFCD00038977 | [MOL File]
33645-24-8.mol | [Molecular Weight]
245.703 |
| Chemical Properties | Back Directory | [Melting point ]
128.0 to 132.0 °C | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [form ]
powder to crystal | [color ]
White to Almost white | [InChI]
InChI=1/C11H15NO3.ClH/c1-8(13)10(12)11(14)15-7-9-5-3-2-4-6-9;/h2-6,8,10,13H,7,12H2,1H3;1H/t8-,10+;/s3 | [InChIKey]
IDZGTFSDZJVMSD-YFYURQRANA-N | [SMILES]
C(OCC1=CC=CC=C1)(=O)[C@H]([C@H](O)C)N.[H]Cl |&1:10,11,r| | [CAS DataBase Reference]
33645-24-8 |
| Hazard Information | Back Directory | [References]
[1] Charles M, et al. Serine and Threonine Schiff Base Esters React with β-Anomeric Peracetates in the Presence of BF3·Et2O to Produce β-Glycosides. Journal of Carbohydrate Chemistry, 2010; 29. | [Uses]
L-threonine benzyl ester hydrochloride salt was reacted with the diaryl ketimine bis-(4-methoxyphenyl)-methenamine in CH3CN at rt to form the more nucleophilic L-threonine Schiff base (Benzyl N-bis (4-methoxyphenyl)methylene-L-threoninate) in excellent yield. In addition, L-threonine Schiff base, acting as glycosyl acceptor, reacted at rt with simple sugar peracetate donor with BF3·OEt2 promotion to provide the corresponding L-threonine O-linked glycosides in excellent yields and purities[1].
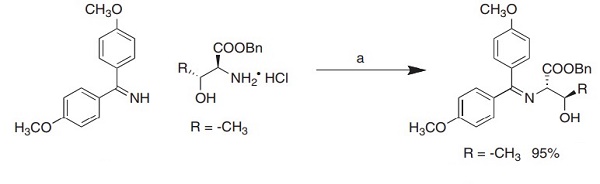
| [Preparation]
The benzyl ester hydrochloride salt, L-Threonine Benzyl Ester Hydrochloride, was prepared from L-threonine and PhCH2OH in the presence of p-toluenesulfonic acid[1]. Specifically, L-Threonine (4.0 g, 34 mmol) and pTsOH acid monohydrate (7.10 g, 38 mmol) were reacted with benzyl alcohol (40 mL) in 50 mL dry benzene using a Dean-Stark apparatus according to the procedure of Petursson and Bald-win. Yield:71.3% |
|
|