| Identification | Back Directory | [Name]
p-hydroxyphenyl methacrylate | [CAS]
31480-93-0 | [Synonyms]
Einecs 250-652-7
4-methacryloxyphenol
4-hydroxyphenyl Methacrylate
p-hydroxyphenyl methacrylate
4-Hydroxyphenyl methacrylate,97%
Methacrylic acid=4-hydroxyphenyl ester
2-Methylpropenoic acid 4-hydroxyphenyl ester
2-Methyl-2-Propenoic Acid 4-Hydroxyphenyl Ester
2-Propenoic acid, 2-methyl-, 4-hydroxyphenyl ester | [EINECS(EC#)]
250-652-7 | [Molecular Formula]
C10H10O3 | [MDL Number]
MFCD00227728 | [MOL File]
31480-93-0.mol | [Molecular Weight]
178.18 |
| Hazard Information | Back Directory | [Description]
p-Hydroxyphenyl methacrylate is a monomer that belongs to the class of benzene derivatives. | [Chemical Properties]
white to light yellow to light orange powder to crystal | [Uses]
p-Hydroxyphenyl methacrylate can be used as a precursor for a carbon nanotube. This material has been studied for its adsorption kinetics, dose-dependent, and directional properties. It has been shown to have chloride ion sensitive adsorption properties. It also has an isotherm that is dependent on temperature and irradiation with UV light. When irradiated with UV light, it shows gel matrix formation and crosslinking. p-Hydroxyphenyl methacrylate can also be used in membranes and water surface gels. | [Preparation]
The preparation of p-hydroxyphenyl methacrylate is as follows:Add 3992ml of n-hexane to the 5L four-necked flask equipped with stirring paddle, condenser tube and thermometer, turn on stirring, add 83.78g of p-hydroxyphenol to the system, slowly add 0.02g of polymerization inhibitor p-benzoquinone to the reaction system, add 102.36 g of g basic ion exchange resin, adjust pH=7-8, cool down to -19°C, slowly add 102.36g vinyl methacrylate dropwise to the reaction system, keep the reaction for 6h, monitor the reaction progress, take samples to detect the reaction is complete and then go to the reaction system 1000 ml of water was added, and the mixture was stirred for extraction and phase separation. The organic phase was dried, concentrated under reduced pressure and evaporated to dryness to obtain 127 g of 2-methyl-2-acrylic acid-4-hydroxyphenyl ester product, with a yield of 93.68%.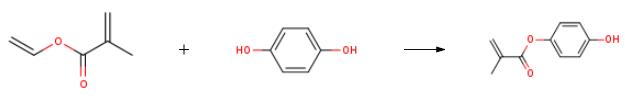
|
|
|