| Identification | Back Directory | [Name]
4-bromo-2,5-difluorobenzoic acid | [CAS]
28314-82-1 | [Synonyms]
4-bromo-2,5-difluorobenzoic acid
2,5-Difluoro-4-broMobenzoic acid
Benzoic acid, 4-broMo-2,5-difluoro-
4-bromo-2,5-difluorobenzoic acid ISO 9001:2015 REACH | [Molecular Formula]
C7H3BrF2O2 | [MDL Number]
MFCD00844105 | [MOL File]
28314-82-1.mol | [Molecular Weight]
237 |
| Chemical Properties | Back Directory | [Boiling point ]
285.0±40.0 °C(Predicted) | [density ]
1.872±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
wooly powder | [pka]
2.70±0.10(Predicted) | [color ]
Creamy peach | [InChI]
InChI=1S/C7H3BrF2O2/c8-4-2-5(9)3(7(11)12)1-6(4)10/h1-2H,(H,11,12) | [InChIKey]
YWZSTHLOAZTDFJ-UHFFFAOYSA-N | [SMILES]
C(O)(=O)C1=CC(F)=C(Br)C=C1F |
| Hazard Information | Back Directory | [Uses]
4-Bromo-2,5-difluorobenzoic acid is a synthetic chemical compound that is widely used in scientific research. It is a versatile compound that can be used in many different laboratory experiments. It is also a fluorinated benzoic acid derivative and can be used as a reagent in a variety of organic and inorganic reactions.
| [Synthesis]
Freshly titrated n-butyl lithium (27.0 ml, 1.39 M in hexanes) is added slowly (over about 30 minutes) to a -78°C solution of diethyl ether (90 ml) containing 1,4-dibromo-2,5-difluorobenzene (10.22 g, 0.038 mol). The resulting yellow solution is stirred at -78°C for 2 hours to give a yellow suspension. Several pellets (~10) of dry ice are added to the suspension, which is then allowed to warm slowly to room temperature as it degasses (approximately 40 minutes). The resulting suspension is acidified with a 1 M aqueous solution of hydrochloric acid (500 ml), and the product extracted with diethyl ether (5 x 200 ml). The combined organics are washed with water (4 x 100 ml) and filtered. The ether solution is concentrated to approximately 200 ml under reduced pressure, and the product extracted into a saturated aqueous solution of sodium bicarbonate (3 x 200 ml). The combined aqueous extracts are washed with methylene chloride (3 x 100 ml) and acidified with hydrochloric acid. The product is extracted with diethyl ether (3 x 200 ml), and the combined organic extracts washed with water (2 x 200 ml), dried over magnesium sulfate, and concentrated under reduced pressure to give 4-Bromo-2,5-difluorobenzoic acid as a pale yellow solid.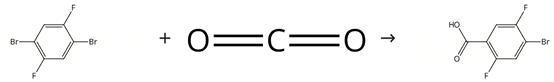 Figure Synthesis of 4-Bromo-2,5-difluorobenzoic acid Figure Synthesis of 4-Bromo-2,5-difluorobenzoic acid |
|
|