| Identification | Back Directory | [Name]
Homoharringtonine | [CAS]
26833-87-4 | [Synonyms]
HHT
HHT2
ORW2
SKR3
ACVRL1
ACVRLK1
CGX 635
Myelostat
NSC 141633
Ceflatonin
Omacetaxine
HMoharringtonine
HOMOHARRINGTONIN
HOMOHARRINGTONINE
HOMOHARRINGTONINUM
HOMOHARRINGTONINE(P)
Homoharringtonine CP
HoMoharringtonine (8CI)
Omacetaxine Mepesuccinate
OMacetaxine (HoMoharringtonine)
(2'R,3S,4S,5R)-(-)-Homoharringtonine
ANTI-ACVRL1 antibody produced in mouse
ALK1 (144-end), active, GST tagged human
4-methylpentyl)butanedioate(ester), [3(R)]-
Cephalotaxine, 4-methyl2-hydroxy-2-(4-hydroxy-
Anti-ACVRL1 (C-terminal) antibody produced in goat
(12S)-12-Hydroxy-5,8,10-heptadecanetricarboxylic acid
(2R)-2-hydroxy-2-(4-hydroxy-4-Methylpentyl)butanedioate
cephalotaxine 4-methyl 2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate
4-methyl-cephalotaxin2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate(e
cephalotaxine,4-methyl(2r)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioa
Cephalotaxine (R)-2-(methoxycarbonylmethyl)-2,6-dihydroxy-6-methylheptanoate
CEPHALOTAXINE 4-METHYL (2R)-2-HYDROXY-2-(4-HYDROXY-4-METHYLPENTYL)BUTANEDIOATE
CEPHALOTAXINE, 4-METHYL-2-HYDROXY-2-[4-HYDROXY-4-METHYLPENTYL] BUTANEDIOATE ESTER
Cephalotaxine, 4-methyl (2R)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate (ester)
(2'R,3S,4S,5R)-(-)-hoMoharringtonine, (-)-hoMoharringtonine, hoMoharringtonine, hoMoharrigtonine, harringtonine, HHT, HoMoharringtonine | [Molecular Formula]
C29H39NO9 | [MDL Number]
MFCD01075772 | [MOL File]
26833-87-4.mol | [Molecular Weight]
545.62 |
| Chemical Properties | Back Directory | [Appearance]
Off-white Cryst | [Melting point ]
144-146 C | [Boiling point ]
619.03°C (rough estimate) | [density ]
1.2395 (rough estimate) | [refractive index ]
1.6290 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
DMSO: soluble20mg/mL, clear | [form ]
powder | [pka]
11.60±0.29(Predicted) | [color ]
white to beige | [biological source]
mouse | [optical activity]
[α]/D -120 to -140°, c = 1 in chloroform-d | [InChIKey]
DJIVDDPFKDEQIR-XSEHADPMSA-N |
| Hazard Information | Back Directory | [Chemical Properties]
Off-white Cryst | [Definition]
ChEBI: A cephalotaxine-derived alkaloid ester obtained from Cephalotaxus harringtonia; used for the treatment of chronic or accelerated phase chronic myeloid leukaemia. | [Biological Activity]
Inhibitor of protein synthesis. Blocks elongation phase of translation by binding to the 60-S ribosome subunit. Antileukemic. | [Description]
Omacetaxine mepesuccinate (also known as homoharringtonine) was
approved by the US FDA in October 2012 for the treatment of patients with
chronic or accelerated phase chronic myeloid leukemia (CML) with resistance
or intolerance to at least two tyrosine kinase inhibitors (TKIs).
Omacetaxine is a protein synthesis inhibitor that was studied in the 1970s for the treatment of acute myeloid leukemia (AML) and in the 1990s for
CML. Emergence of resistance to first- and second-generation TKIs has lead
to renewed interest in omacetaxine due to its differentiated mode of action.
Omacetaxine acts on the initial step of protein translation andresults in the rapid loss of a number of short-lived proteins that regulate proliferation and cell survival. Omacetaxine induces apoptosis and shows in vitro activity in anumberof leukemia cell lines andinmurine leukemiamodels. Omacetaxineis
a naturally occurring alkaloid isolated from Cephalotaxus coniferous shrubs that are indigenous to Asia. Extracts of the bark have been used by practitioners of traditional Chinese medicine for the treatment of cancer. Although omacetaxine could be isolated directly from bark and roots, a more efficient approach is semi-synthesis by esterification of the abundant biosynthetic precursor cephalotaxine, which can be extracted from leaves rather than nonrenewable sources. Esterification is carried out with an activated ester in which the diol side-chain is protected as a tetrahydropyran; after ester formation, the diol is released in two steps under mild conditions. | [Physical properties]
Appearance: an almost white or pale yellow crystalline powder or an amorphous friable solid. It has the hygroscopic nature. It darkens on exposure to light. Solubility: easily soluble in chloroform, ethanol and methanol, and slightly soluble in ether and water. Melting point: 143–147?°C. | [Originator]
Chinese Academy of Medical Sciences/ChemGenex (China/United States) | [History]
In China, especially in Fujian, etc., doctors have treated tumor with Cephalotaxus harringtonia long ago. In the early 1970s, American scientists Powell et?al. isolated and identified the alkaloids of the Cephalotaxus plant and studied its antitumor activity. One type of the alkaloids (Cephalotaxine ester, including harringtonine, homoharringtonine, isoharringtonine, deoxyharringtonine, and pseudodeoxyharringtonine) was found to have the effect of inhibiting the proliferation of mouse leukemia cells . Chinese scientists also isolated a large number of harringtonine and homoharringtonine and used them to treat leukemia. Then, the United States and other developed countries’ scientists carried out the Phase I and II clinical research and toxicology research of homoharringtonine. | [Indications]
This product is recorded in the Pharmacopoeia of the People’s Republic of China (2015).
Its main dosage form is homoharringtonine injection, which is mainly used for
the treatment of chronic myelocytic leukemia and acute myeloid leukemia.
| [Brand name]
Synribo | [Biochem/physiol Actions]
Homoharringtonine is a cephalotaxine ester that is also known as (HHT; 4-methyl (2R)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate). In gefitinib-resistant lung cancer cells, homoharringtonine promotes apoptosis and prevents signal transducer and activator of transcription 3 (STAT3) through IL-6 (interleukin-6) /JAK1 (janus kinase 1)/STAT3 signal pathway. It plays an important role in the treatment of malaria. | [Pharmacology]
Induce apoptosis: Homoharringtonine was found to activate HL-60 cell apoptosis through caspase-3 mediated Bcl-2-Bax, -MAPK pathway .
3. Induce cell differentiation: Homoharringtonine could induce HL-60 cell differentiation by downregulating the expression of CD44 gene, thereby increasing p27 and p21 expression and inhibition of cyclin E activity.
Structure-Activity Relationship (SAR) The methyl acetate group of 2′ position in the C-3 acyl side chain is an active essential group. R group plays a role in regulating the polarity of the molecule, and the size of the R group can affect the molecular activity. The olefinic carbon instead of the 2′ position chiral carbon is still active . | [Anticancer Research]
This compound is isolated from Cephalotaxus harringtonia. A racemic mixture ofharringtonine and homoharringtonine is used for acute and chronic myelogenousleukemia (Shoeb 2006). | [Clinical Use]
Synribo® (Omacetaxine mepesuccinate) was approved by the FDA for the treatment of adult patients
with chronic or accelerated phase chronic myeloid leukemia (CML) exhibiting resistance or intolerance
to tyrosine kinase inhibitors (TKI’s). Omacetaxine mepesuccinate inhibits protein synthesis and
prevents aminoacyl-tRNA binding during the elongation phase and targets myeloma-promoting
molecules Mcl-1, XIAP, and β-catenin, which are particularly important in the survival of
myeloma cells. Omacetaxine mepesuccinate is also known as homoharringtonine, an alkaloid
originally discovered and structurally identified from Cephalotaxus harringtonia, which occurs
naturally in Japan and eastern Asia. | [Synthesis]
Because of its leukemic activity and interesting chemical structure,
the core and ester side chains of the cephalotaxine alkaloids have been the focus of numerous synthetic
studies. However, large-scale production often utilizes a semisynthetic route which relies upon
cephalataxine (CET) derived from natural sources coupled with a synthetically obtained ester side
chain. The challenges associated with direct esterification of cephalotaxine with the
homoharringtonine and other related ester side chains are the basis of ongoing research aimed at
identification of improved side-chain coupling methods. The most likely process-scale synthetic
route features the coupling of the homoharringtonine side chain with the cephalotaxine core, and a
subsequent conversion of the |á-hydroxy moiety to a bridged heterocyclic species followed by ring
opening after coupling to give the active homoharringtonine product, and this is described in the scheme below.
The method for large scale synthesis of homoharringtonine begins with derivatization of commercial
5-bromo-2-methyl-pent-2-ene (100) with diethyl oxalate and the pre-formed enolate of methyl acetate
(101), generating diester 102 in 50% overall yield. Acid-promoted pyran formation,
followed by universal ester saponification and selective re-esterification provided the desired racemic
pyran acid 103. Activation of acid 103 with 2,4,6-trichlorobenzoyl chloride (104) and subsequent addition of quinine (105) led to a mixture of diastereomers 106a/106b (1:1) in 84% yield, which were
separable by chromatographic methods. Diastereomer 106a was then carried on to the synthesis of
homoharringtonine (Omacetaxine mepesuccinate) as described in the scheme below.
From isomer 106a, hydrogenolysis with Pd/C and H2 provided acid 107 in 50% yield. Activation of
107 with 2,4,6-trichlorobenzoyl chloride 104 followed by addition of cephalotaxine (CET) (108)
provided the desired cephalotaxine-coupled product 109 in 43% yield. Sequential treatment with
HBr/HOAc and 5% aqueous NaHCO3 completed the synthesis, providing omacetaxine mepesuccinate
(XVII) in 41% yield (over two steps). | [storage]
4°C, protect from light |
| Questions And Answer | Back Directory | [Alkaloids]
Homoharringtonine (HHT) is an alkaloid isolated from the total alkaloid of Cephalotaxus. It is a highly effective antineoplastic agent in our country. It has been clinically proved to be well effective against acute myeloid leukemia, acute monocytic leukemia, erythroid leukemia and other acute non-lymphocytic leukemia and chronic myeloid leukemia, but it has a strong cytotoxicity and can also lead to cardiac toxicity and bone marrow suppression, limiting the increase in chemotherapy dose.
The above information is edited by Chemicalbook. | [Physical and chemical properties]
It appears as white crystalline or yellowish amorphous solid with bitter taste. It has a melting point of 141-143 ° C, density of 1.33g/cm3, boiling point of 713.1 ° C and a flash point of 385.1 °C. It has hygroscopicity. Its color will turn deep in case of light, being easily soluble in methanol, ethanol, chloroform, but slightly soluble in water and ether. | [Plant source]
Homoharringtonine is an alkaloid derived from cephalotaxus or its genera. There are 9 species of Cephalotaxus in Cephalotaxus, among which 8 species are found in China. This genus contains a variety of alkaloids, that is, harringtonine, and homoharringtonine, isohetonium and isochitraline deoxyspermatine base; in addition, there is also non-ester base form of cephalotaxine (That is, harringtonine). | [Cephalotaxus fortune]
Cephalotaxus fortune belongs to Cephalotaxus genus plant, which only contains Cephalotaxus, one genus including 9 species. It is mainly distributed in the south of the Yangtze River and in Hubei, Sichuan, Shaanxi, Gansu and other places. There are 7 species and 3 varieties in China, which are mainly distributed in the south of Yangtze River. Cephalotaxus contains alkaloids in branches, leaves, roots and seeds especially having high alkaloid content at leaves and seeds, including cephalotaxine type (Cephalotaxine type) alkaloids and anti-cancer are closely related, the content is also high, being the major component of Cephalotaxus, harringtonine, isoflavone, homoharringtonine and deoxy-oxytocin. It has been isolated of two new anticancer alkaloids, named Neoharringtonine and Anhydroharringtonine from Cephalotaxus fortunei in recent years.
Cephalotaxus fruit is used for removing food retention, anthelmintic. In the early 1970s, the United States scientists Powell et al had found that Harringtonine compounds had effects of inhibiting the growth of mouse leukemia cells, drawing wide study and attention from researchers on the alkaloid component.

Fig. 1 is a photograph of leaves and fruits of Cephalotaxus fortunei. | [Analytical method]
High-performance liquid chromatography (US HP 1100); UV scanner (UV300-type). Column: Hypersil BDS (4.0 mm × 250 mm, 5 Lm); mobile phase: 0.0008 mol/L-1 ammonium carbonate solution-methanol (42:58); the detection wavelength was 290 nm and the flow rate was 110 mL/min-1. Injection volume: 20 μL. | [Plant extracts]
The root, stem and bark of Cephalotaxus fortunei can be made into coarse powder; further added with alcohol to extract the solution. The obtained solution is separated by column chromatography with aluminum hydroxide, and then the silica gel column is used to separate the eluents. Homoharringtonine and harringtonine can be successively obtained. | [Synthesis method]
The homoharringtonine is synthesized using δ-methyl-δ-caprolactone (1) and ethanol as starting materials via ring opening and elimination of ethyl 5-methyl-4-hexenoate (2), 2 has condensation reaction with diethyl oxalate to give diethyl 2-(3-methyl-2-butenyl) malonate (3), 3 has β-decarboxylation reaction with aqueous dimethylsulfoxide in the presence of sodium chloride to generate 6-methyl-2-oxo-5-heptenoic acid ethyl ester (4); 4 is subject to alkaline hydrolysis, oxalylation and further acylation of cephalotaxine to obtain 6-methyl-2-Oxo-5-heptenoic acid cephalomannine ester (6) and then subjected to a Rafalmacky reaction to give methyl 2-hydroxy-2-(4-methyl-3-pentenyl) succinate Cephalotaxine ester (7), 7 is subject to mercury hydroxylation and mercury removal reaction to obtain the end product, homoharringtonine. The reaction scheme is as follows:
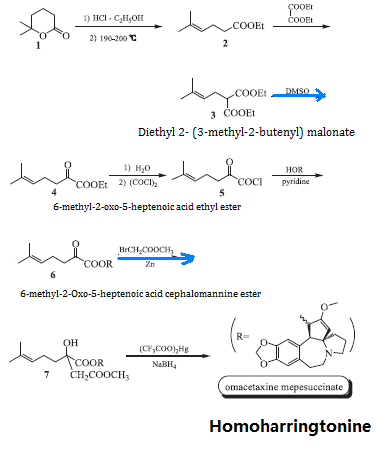
Figure 2 is a chemical reaction route map for the laboratory synthesis of homoharringtonine. | [Pharmacological activity]
This product has bitter taste and high toxicity and has effect on the kidney, liver and lung. It can eliminate stasis to subdue swelling as well as reducing phlegm and resolving masses.
Inhibition of leukemia cells by homoharringtonine is mediated by induction of leukemic cells into normal differentiation. One of its molecular pharmacological pathways may be through inhibition cell Na-K-ATPase activity and further inhibition of activities of tumor cell membrane thymidine carrier and protein kinase C. It has also been reported that the harringtonine and homoharringtonine can activate the apoptosis mechanism of tumor cells, and it is believed that these compounds can inhibit tumor growth partly through tumor cell programmed death. | [uses]
Homoharringtonine mainly inhibit leukemia cell DNA and protein synthesis, dissolving the nucleus and causing its necrosis with remarkable nucleolus lesions. It also inhibits the cell in any phases within the proliferation cycle; the most significant effect is the S phase; it can also inhibit and destroy G2 phase cells so that most of the G2 phase cells were inhibited from entering into the M phase, being the cell cycle non-specific drugs. It is used for the treatment of acute monocytic leukemia, acute myeloid leukemia, acute promyelocytic leukemia and red blood leukemia, acute myelogenous leukemia and malignant lymphoma. It is also of certain efficacy against the malignant lymphoma and polycythemia Vera as well as some other types of leukemia. | [Toxicology]
Toxicity of harringtonine (HA) and homoharringtonine (HO) was studied in mice, rabbits and dogs. Rabbits and dogs, after five or seven days of continuous administration, have their main target organ of toxicity be gastrointestinal, cardiac and hematopoietic organs. Most of the toxic deaths can be attributed to cardiac dysfunction. With the lethal dose, there are also individual cases of mild to moderate liver and kidney damage. Intermittent repeated administration of a total of three courses causes no other significant toxicity, but the heart and hematopoietic study exhibit mild to moderate cumulative toxicity with the toxicity correlating well with the dose size and is completely reversible after drug withdrawal. No significant late-onset toxicity was observed during at least six weeks of observation, and no significant differences were found between the two species in terms of inter-individual, inter-individual or gender-related toxicities. The toxic dosage of HA and HO were 0.45 mg/kg/day × 5 and 0.16 mg/kg/day × 7, respectively. The acute LD_ (50) (± S.E.) in mice was 4.17 ± 0.30 and 3.17 ± 0.19mg/kg, respectively. HA, compared with HO, has no significant toxicity difference in nature while the extent of HO is significantly greater than HA. | [Metabolism]
1. In vivo metabolism: 2 hours after intravenous injection, the concentration of the products undergoes rapid decline in each tissue while the decline rate in the bone marrow is slower. The half-life is 3~50 min and the metabolism in the body are more active with the main metabolism processing in the liver, but its metabolite is not clear. When being excreted by the kidneys and biliary tract, a small amount is subject to excretion through the feces. In the discharge, the prototype drug accounted for 1/3. At 24 hours after administration, the excretion was about 50% of the total dose, of which 42.2% was excreted in the urine and 6.3% in the feces.
2. Clinical Metabolism: Homoharringtonine is in two-compartment distribution inside the body with the pharmacokinetics varying significantly in different individual.
3. Chemotherapy Metabolism: The parameters related to chemotherapy efficacy were K12/K21, the difference between the effective and ineffective groups was very significant (P <0.01).

(n=41) Due to that in one case, the tests of P170, mdrl and sensitization are all positive, indicating that this patient is drug-tolerant, thus not included in this statistic. | [Side effects]
1. Gastrointestinal reactions: anorexia, dry mouth, nausea, vomiting, abdominal pain, diarrhea; a small number of patients can get liver damage with very few people having oral ulcers.
2. Cardiac toxicity: seen in large doses and rapid injection or patients originally having heart disease; manifested as arrhythmia, myocardial damage, palpitations sinus tachycardia, atrial or ventricular premature beats and other cardiac toxicity and hypotension. In some cases, it can be seen of myocardial damage. Patients of organic heart disease, arrhythmia, liver and kidney dysfunction should take with caution.
3. Bone marrow suppression: mainly exhibits as leukopenia. Moreover, the product has inhibitory effect against different series of bone marrow hematopoietic cells, stronger inhibition against granulocyte series, followed by red blood cell series. The inhibition of megakaryocyte series is lighter.
4. Other: individual patients can get hair loss, rash and so on. | [Usage amount]
This product has no cross-resistance with Ara-C and mercaptopurine. Intramuscular injection or oral absorption is slow and incomplete. It is mainly administrated through intravenous injection. After intravenous injection, the highest concentration is found in bone marrow, followed by kidney, liver, lung, spleen, heart and stomach, followed by the intestine and muscle and brain tissue has the lowest level. | [Precautions]
1. To avoid fetal death and congenital malformations, pregnant women and lactating women should take with caution.
2. As the elderly patients have poor tolerance to chemotherapy, and thus administration of this product should also be accompanied with strengthened supportive therapy, and close observation of various adverse reactions.
3. The diagnosis of interference: a large number of leukemia cells damage upon leukemia; destruction will become more severe upon using this product; the urine and uric acid concentration in the blood may be increased.
4. Cardiovascular disease: rapid intravenous infusion or long-term sustained or repeated administration will produce a variety of cardiac toxicity. Animal experiments show that high doses of homoharringtonine intravenously can significantly reduce the coronary blood flow, so upon FDA, the intravenous infusion rate should be slow. For patients of original arrhythmia and various types of organic cardiovascular disease should take with caution; for patients of serious or frequent arrhythmias and organic cardiovascular disease, they should not use this product. The above cardiotoxicity, except in very severe cases, will generally disappear after disabling this product.
5. In the following cases, it should be used with caution: bone marrow function was significantly inhibited or blood phase exhibited severe neutropenia or thrombocytopenia, arrhythmia and have other organic cardiovascular disease history; liver dysfunction or renal dysfunction, gout or having uric acid salt kidney history.
6. During the treatment period, it should be checked regularly of the following stuffs:
① peripheral blood, weekly follow-up for white blood cell count and classification, platelet, hemoglobin for 1 or 2 times; For patients undergoing a sudden decline in blood cells in the short term, they should be observed daily of the blood phase.
② liver function, including serum 1min bilirubin, total bilirubin, alanine aminotransferase and so on.
③ cardiac signs and ECG examination. | [Storage methods]
Shade, sealed, stored in the shade. |
|
|