| Identification | Back Directory | [Name]
1-ethylcyclopentyl ester | [CAS]
266308-58-1 | [Synonyms]
1-ethylcyclopentyl ester
1-Ethylcyclopentyl methacrylate
1-ethylcyclopentyl ester 266308-58-1
Methacrylic Acid 1-Ethylcyclopentyl Ester
(1-ethylcyclopentyl) 2-methylprop-2-enoate
1-Ethylcyclopentyl methacrylate 266308-58-1
2-Propenoic acid, 2-methyl-, 1-ethylcyclopentyl ester
1-Ethylcyclopentyl Methacrylate (stabilized with MEHQ)
EtCPMA 1-Ethylcyclopentyl methacrylate ArF monomers | [Molecular Formula]
C11H18O2 | [MDL Number]
MFCD23703079 | [MOL File]
266308-58-1.mol | [Molecular Weight]
182.259 |
| Chemical Properties | Back Directory | [Boiling point ]
225.9±9.0 °C(Predicted) | [density ]
0.95±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,2-8°C | [form ]
clear liquid | [color ]
Colorless to Light yellow to Light orange | [InChI]
InChI=1S/C11H18O2/c1-4-11(7-5-6-8-11)13-10(12)9(2)3/h2,4-8H2,1,3H3 | [InChIKey]
FMEBJQQRPGHVOR-UHFFFAOYSA-N | [SMILES]
C(OC1(CC)CCCC1)(=O)C(C)=C | [CAS DataBase Reference]
266308-58-1 |
| Hazard Information | Back Directory | [Chemical Properties]
Colorless to Light yellow to Light orange clear liquid. | [Physical properties]
Lan et al. use ellipsometry and fluorescence to study how the glass transition temperature (Tg) is affected by confinement in silica-supported films of low and high MW poly(1-ethylcyclopentyl methacrylate) (PECPMA) and poly(methyl methacrylate) (PMMA). With decreasing nanoscale thickness, Tg is nearly invariant for high MW (Mn =22.5, 188 and 297 kg/mol) PECPMA but decreases for low MW PECPMA, with Tg-Tg, bulk =-7 to 8 C in a 27-nm-thick film (Mn=4.1 kg/mol) via ellipsometry and 15 ℃ in a 17- nm-thick film (Mn =4.9 kg/mol) via fluorescence. Fluorescence studies using a 20-nm-thick dye-labelled layer in multilayer, bulk PECPMA films reveal a much greater perturbation to Tg in the free-surface layer for low MW PECPMA, which propagates tens of nanometers into the film[2].
| [Uses]
Used as Intermediate in organic synthesis. | [Application]
1-ethylcyclopentyl ester is commonly used as a photoresist monomer.
| [Preparation]
The preparation of 1-ethylcyclopentyl ester is as follows:It is determined that the 14 and 6 kettles of workshop 106 are thoroughly cleaned, and dichloromethane (300kg), 1-ethylcyclopentanol (20kg, 175mol), triethylamine (50kg, 494mol), 4-dimethylaminopyridine ( 0.2kg, 1.64mol) and phenothiazine (0.4kg, 2.01mol) were added to the 14 kettle. Weigh methacryloyl chloride (27.4kg, 262mol) into the 14 kettle header tank. Use frozen brine to cool down to -5 ~ 0 . The temperature is controlled at 0±5°C, the methacryloyl chloride in the high-level tank is added dropwise to the kettle, and the feed liquid in the kettle is kept at 0-5°C for 2 hours for reaction. Start sampling and testing after 2 hours of incubation, the raw material (1-ethylcyclopentanol) is less than 2.0%Add water (100kg) to the 6 kettle, transfer the feed liquid in the 14 kettle to the 6 kettle, quench, stir for 30 minutes, and let stand for 30 minutes. The kettle liquid appeared layering phenomenon, the upper water phase was separated, the lower organic layer was washed twice with 15% aqueous sodium hydroxide solution (100kg water and 15kg liquid caustic soda), and the organic layer was separated. The organic layer was washed twice with water (100 kg), and the organic layer was separated. Add anhydrous sodium sulfate (10kg) to the organic layer, dry, filter with suction, add phenothiazine (0.2kg), control the temperature to 20-25°C, and concentrate under reduced pressure to obtain the crude product. Sampling for GC detection shows that the residual solvent is less than 1.0%. The crude oil pump vacuum distillation (oil pump, 1mmHg column, main distillate temperature 4244 , external temperature 80-85 ) obtains a total of 18.5kg, 101.5mol, and 58% content is greater than 98%.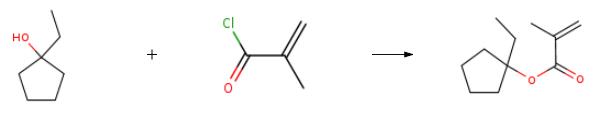
| [storage]
Being kept in dry, clean warehouse with well ventilation.
Avoid exposing to light and heating.
Protecting the product from leakage, rain and insolation during transportation.
| [References]
[1] Guozhen Zhang. “A Combined Computational and Experimental Study of Copolymerization Propagation Kinetics for 1-Ethylcyclopentyl methacrylate and Methyl methacrylate.” Macromolecular Theory and Simulations 25 3 (2016): 263–273.
[2] Tian Lan, John M. Torkelson. “Methacrylate-based polymer films useful in lithographic applications exhibit different glass transition temperature-confinement effects at high and low molecular weight.” Polymer 55 5 (2014): Pages 1249-1258.
|
|
|