| Identification | Back Directory | [Name]
Tolmetin | [CAS]
26171-23-3 | [Synonyms]
C07149
McN 2559
Tolmetin
Tolmetine
TOLMETINUM
Tolmetin Solution, 100ppm
1-Methyl-5-p-toluoylpyrrole-2-acetic acid
1-Methyl-5-(p-toluoyl)-1H-pyrrole-2-acetic acid
5-[(p-Tolyl)carbonyl]-1-methylpyrrole-2-acetic acid
1-Methyl-5-(4-methylbenzoyl)-1H-pyrrole-2-acetic acid
1H-Pyrrole-2-acetic acid, 1-methyl-5-(4-methylbenzoyl)-
2-[1-Methyl-5-(4-methylbenzoyl)-pyrrol-2-yl]acetic acid
2-(1-Methyl-5-(4-Methylbenzoyl)-1H-pyrrol-2-yl)acetic acid
2-[1-methyl-5-[(4-methylphenyl)-oxomethyl]-2-pyrrolyl]acetic acid
2-{1-Methyl-5-[(4-Methylphenyl)carbonyl]-1H-pyrrol-2-yl}acetic acid | [EINECS(EC#)]
247-497-2 | [Molecular Formula]
C15H15NO3 | [MDL Number]
MFCD00599595 | [MOL File]
26171-23-3.mol | [Molecular Weight]
257.28 |
| Hazard Information | Back Directory | [Description]
An antiinflammatory, analgesic, and antipyretic that is as efficacious as
moderate doses of aspirin and better tolerated. Tolmetin produces
a number of adverse effects including epigastric pain, dyspepsia,
nausea, and vomiting. Tolmetin is approximately 99% plasma
protein bound, yet does not interfere with concurrent treatment
with oral hypoglycemics. Tolmetin has been found to be effective in the treatment of osteoarthritis and rheumatoid arthritis. | [Originator]
Tolectin,McNeil,US,1976 | [Uses]
inhibits synthesis of prostaglandins and
exhibits expressed analgesic, anti-inflammatory, and fever-reducing properties. It is used
for relieving weak to moderate pain in rheumatoid arthritis and osteoarthritis. | [Definition]
ChEBI: A monocarboxylic acid that is (1-methylpyrrol-2-yl)acetic acid substituted at position 5 on the pyrrole ring by a 4-methylbenzoyl group. Used in the form of its sodium salt dihydrate as a nonselective nonsteroidal anti-inflammatory drug. | [Indications]
Tolmetin (Tolectin) is indicated for the relief of osteoarthritis,
rheumatoid arthritis, ankylosing spondylitis,
and moderate pain. It is ineffective in gouty arthritis for
unknown reasons.Tolmetin can inhibit both COX-1 and
COX-2 but has a moderate selectivity for COX-1. The
most frequently reported side effects are GI disturbance
and CNS reactions (e.g., headache, asthenia, and
dizziness). These effects are less frequently observed
than after aspirin or indomethacin use. Blood pressure
elevation, edema, and weight gain or loss have been
associated with tolmetin administration. Tolmetin metabolites
in urine have been found to produce pseudoproteinuria
in some laboratory tests. | [Manufacturing Process]
5-(p-Toluoyl)-1-methylpyrrole-2-acetonitrile - To a cooled suspension of 26.6 g
(0.2 mol) aluminum chloride in 80 ml dichloroethane is added dropwise 30.8 g
(0.2 mol) p-toluoyl chloride. The resulting solution is added dropwise to a
solution of 1-methylpyrrole-2-acetonitrile in 80 ml dichloroethane cooled
externally with an ice bath. After the addition, the resulting solution is stirred
at room temperature for 20 minutes and then refluxed for 3 minutes. The
solution is poured into ice acidified with dilute hydrochloric acid. The organic
and aqueous fractions are separated. The aqueous fraction is extracted once
with chloroform.
The organic fractions are combined and washed successively with N,N_x0002_dimethyl-1,3-propanediamine, dilute hydrochloric acid, saturated sodium
bicarbonate solution and saturated sodium chloride solution. The organic
fraction is dried over anhydrous magnesium sulfate. The solvent is then
evaporated off. Upon trituration of the residue with methanol, a solid
crystallizes, 5-(p-toluoyl)-1-methylpyrrole-2-acetonitrile, which is removed by
filtration and purified by recrystallization from benzene.
Additional product is isolated from the mother liquors which are combined,
concentrated in vacuo and the resulting oily residue column chromatographed
on neutral alumina using hexane, benzene and ether as successive solvents.
The product is isolated by concentrating in vacuo the first few major
compound-bearing fractions (10% ether in benzene). The solids are combined
and recrystallized from methanol and then from benzene-hexane, melting
point 102°C to 105°C.
5-(p-Toluoyl)-1-methylpyrrole-2-acetic acid - A solution of 3.67 g (0.015 mol)
of 5-(p-toluoyl)-1-methylpyrrole-2-acetonitrile, 24 ml of 1 N sodium hydroxide
and 50 ml of 95% ethanol is stirred and refluxed for 24 hours.
The resulting solution is poured into ice acidified with dilute hydrochloric acid.
A white solid precipitates which is extracted into ether. The ether phase is
washed with a saturated solution of sodium chloride and dried over anhydrous
magnesium sulfate. The solvent is evaporated and a white solid, 5-(p-toluoyl)-
1-methylpyrrole-2-acetic acid is obtained which is recrystallized twice from
isopropanol, melting point 155°C to 157°C. | [Therapeutic Function]
Antiinflammatory | [Biological Functions]
Tolmetin (Tolectin) is an antiinflammatory, analgesic,
and antipyretic agent that produces the usual gastric
distress and ulceration observed with NSAIDs.
However, tolmetin is better tolerated than aspirin and
produces less tinnitus and vertigo. Tolmetin is a substitute
for indomethacin in indomethacin-sensitive patients
and is unique among such drugs in that it can be
used to treat juvenile arthritis. | [General Description]
Tolmetin sodium (Tolectin), is an arylacetic acid derivativewith a pyrrole as the aryl group. This drug is well absorbed and has a relatively short plasma half-life (1 hour). It is recommendedfor use in the management of acute and chronicRA. Its efficacy is similar to aspirin and indomethacin, butwith less frequency of the adverse effects and tinnitus associatedwith aspirin. It does not potentiate coumarin-likedrugs nor alter the blood levels of sulfonylureas or insulin.However, tolmetin, and especially its closely related drug,zomepirac (i.e., with a p-chlorobenzoyl group and an additionalmethyl group on the pyrrole ring), can produce a rarebut fatal anaphylactic reaction because of irreversible bindingof their unstable acyl glucuronides. Zomepirac waswithdrawn from market because it is eliminated only via theester-type, acyl glucuronide. It is possible that tolmetin isless toxic in this regard because it undergoes additional hepaticbenzylic hydroxylation via its p-methyl group and isexcreted as its stable ether glucuronide. | [Trade name]
Artrocaptin (Estedi, Spain),
Tolectin (Cilag, Belgium; Janssen-Cilag, Austria; McNeil, USA). | [Mechanism of action]
Tolmetin inhibits both
isoforms of cyclooxygenase with some preference
for COX-1 . | [Pharmacology]
Tolmetin is administered
orally, rectally, or topically (600–1800 mg/d).
The peak plasma concentrations are reached
within 30 to 60 min. Tolmetin shows a high
plasma protein binding of 99% and a biphasic
plasma half-life of 1 to 2 and 5h, respectively. | [Clinical Use]
Tolmetin is a nonsteroidal
anti-inflammatory drug used for the treatment of
mild to moderate pain states in musculoskeletal,
soft-tissue, and joint disorders like rheumatoid
arthritis, osteoarthritis, and gout as well as juvenile
rheumatoid arthritis. | [Synthesis]
Tolmetin, 1-methyl-5-n-tolylpyrrol-2-acetic acid (3.2.61) is synthesized from 1-
methylindole, which is aminomethylated using formaldehyde and dimethylamine, forming
2-dimethylaminomethyl-1-methylindol (3.2.57). The product is methylated by methyl
iodide, giving the corresponding quaternary salt (3.2.58). Reaction of the product with
sodium cyanide gives 1-methylpyrrole-2-acetonitrile (3.2.59), which is acylated at the free |á-position of the pyrrole ring by 4-methylbenzoylchloride in the presence of aluminum
chloride. The resulting 1-methyl-5-n-toluylpyrrol-2-acetonitrile (3.2.60) undergoes further
alkaline hydrolysis, giving corresponding acid, tolmetin (3.2.61) [116¨C118].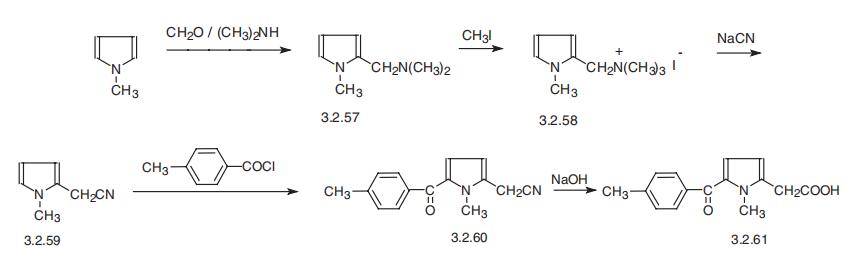
| [storage]
4°C, away from moisture |
|
|