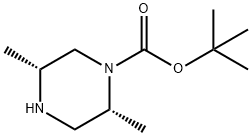
(2R,5R)-2,5-DIMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
|
- $69 - $1880
- Product name: (2R,5R)-2,5-DIMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS: 1240586-48-4
- MF: C11H22N2O2
- MW: 214.3
- EINECS:
- MDL Number:MFCD08686669
- Synonyms:1-Piperazinecarboxylic acid, 2,5-diMethyl-, 1,1-diMethylethyl ester, (2R,5R)-;(2R,5R)-tert-Butyl 2,5-diMethylpiperazine-1-carboxylate;(2R,5R)-2,5-Dimethyl-1-piperazinecarboxylic acid tert-butyl ester;(2R,5R)-2,5-DIMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER;tert-butyl (2R,5R)-2,5-diMethylpiperazine-1-carboxylate
16 prices
Brand
- ACHEMBLOCK
- AK Scientific
- Alichem
- Ambeed
- American Custom Chemicals Corporation
- Apolloscientific
- Chemenu
- Crysdot
- TRC
Package
- 50mg
- 100mg
- 250MG
- 500MG
- 1G
- 5g
- ManufacturerACHEMBLOCK
- Product numberA-538
- Product description(2R,5R)-2,5-Dimethyl-piperazine-1-carboxylicacidtert-butylester 95%
- Packaging1G
- Price$250
- Updated2021-12-16
- Buy
- ManufacturerACHEMBLOCK
- Product numberA-538
- Product description(2R,5R)-2,5-Dimethyl-piperazine-1-carboxylicacidtert-butylester 95%
- Packaging250MG
- Price$100
- Updated2021-12-16
- Buy
- ManufacturerAK Scientific
- Product number4549AA
- Product description(2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate
- Packaging100mg
- Price$153
- Updated2021-12-16
- Buy
- ManufacturerAlichem
- Product number1240586484
- Product description(2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate
- Packaging5g
- Price$865.2
- Updated2021-12-16
- Buy
- ManufacturerAmbeed
- Product numberA259658
- Product description(2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95%
- Packaging100mg
- Price$69
- Updated2021-12-16
- Buy
- ManufacturerAmbeed
- Product numberA259658
- Product description(2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95%
- Packaging250mg
- Price$115
- Updated2021-12-16
- Buy
- ManufacturerAmbeed
- Product numberA259658
- Product description(2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95%
- Packaging1g
- Price$257
- Updated2021-12-16
- Buy
- ManufacturerAmbeed
- Product numberA259658
- Product description(2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95%
- Packaging5g
- Price$893
- Updated2021-12-16
- Buy
- ManufacturerAmerican Custom Chemicals Corporation
- Product numberCCH0003296
- Product description1-N-BOC-2R,5R-DIMETHYL-PIPERAZINE 95.00%
- Packaging500MG
- Price$238.35
- Updated2021-12-16
- Buy
- ManufacturerAmerican Custom Chemicals Corporation
- Product numberCCH0003296
- Product description1-N-BOC-2R,5R-DIMETHYL-PIPERAZINE 95.00%
- Packaging5G
- Price$909.56
- Updated2021-12-16
- Buy
- ManufacturerApolloscientific
- Product numberOR321453
- Product descriptiontert-Butyl(2R,5R)-2,5-dimethylpiperazine-1-carboxylate 95%
- Packaging1g
- Price$580
- Updated2021-12-16
- Buy
- ManufacturerChemenu
- Product numberCM126794
- Product descriptiontert-butyl(2R,5R)-2,5-dimethylpiperazine-1-carboxylate 95%
- Packaging1g
- Price$247
- Updated2021-12-16
- Buy
- ManufacturerCrysdot
- Product numberCD11318536
- Product description(2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95+%
- Packaging1g
- Price$325
- Updated2021-12-16
- Buy
- ManufacturerCrysdot
- Product numberCD11318536
- Product description(2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95+%
- Packaging5g
- Price$765
- Updated2021-12-16
- Buy
- ManufacturerTRC
- Product numberD461288
- Product description(2R,5R)-2,5-Dimethyl-piperazine-1-carboxylicAcidtert-ButylEster
- Packaging50mg
- Price$110
- Updated2021-12-16
- Buy
- ManufacturerTRC
- Product numberD461288
- Product description(2R,5R)-2,5-Dimethyl-piperazine-1-carboxylicAcidtert-ButylEster
- Packaging100mg
- Price$155
- Updated2021-12-16
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| ACHEMBLOCK | A-538 | (2R,5R)-2,5-Dimethyl-piperazine-1-carboxylicacidtert-butylester 95% | 1G | $250 | 2021-12-16 | Buy |
| ACHEMBLOCK | A-538 | (2R,5R)-2,5-Dimethyl-piperazine-1-carboxylicacidtert-butylester 95% | 250MG | $100 | 2021-12-16 | Buy |
| AK Scientific | 4549AA | (2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate | 100mg | $153 | 2021-12-16 | Buy |
| Alichem | 1240586484 | (2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate | 5g | $865.2 | 2021-12-16 | Buy |
| Ambeed | A259658 | (2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95% | 100mg | $69 | 2021-12-16 | Buy |
| Ambeed | A259658 | (2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95% | 250mg | $115 | 2021-12-16 | Buy |
| Ambeed | A259658 | (2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95% | 1g | $257 | 2021-12-16 | Buy |
| Ambeed | A259658 | (2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95% | 5g | $893 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CCH0003296 | 1-N-BOC-2R,5R-DIMETHYL-PIPERAZINE 95.00% | 500MG | $238.35 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CCH0003296 | 1-N-BOC-2R,5R-DIMETHYL-PIPERAZINE 95.00% | 5G | $909.56 | 2021-12-16 | Buy |
| Apolloscientific | OR321453 | tert-Butyl(2R,5R)-2,5-dimethylpiperazine-1-carboxylate 95% | 1g | $580 | 2021-12-16 | Buy |
| Chemenu | CM126794 | tert-butyl(2R,5R)-2,5-dimethylpiperazine-1-carboxylate 95% | 1g | $247 | 2021-12-16 | Buy |
| Crysdot | CD11318536 | (2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95+% | 1g | $325 | 2021-12-16 | Buy |
| Crysdot | CD11318536 | (2R,5R)-tert-Butyl2,5-dimethylpiperazine-1-carboxylate 95+% | 5g | $765 | 2021-12-16 | Buy |
| TRC | D461288 | (2R,5R)-2,5-Dimethyl-piperazine-1-carboxylicAcidtert-ButylEster | 50mg | $110 | 2021-12-16 | Buy |
| TRC | D461288 | (2R,5R)-2,5-Dimethyl-piperazine-1-carboxylicAcidtert-ButylEster | 100mg | $155 | 2021-12-16 | Buy |
Properties
Boiling point :280℃
Density :0.970
Flash point :123℃
storage temp. :2-8°C(protect from light)
pka :8.55±0.60(Predicted)
Density :0.970
Flash point :123℃
storage temp. :2-8°C(protect from light)
pka :8.55±0.60(Predicted)
Safety Information
| Symbol(GHS): | ||||||||
|---|---|---|---|---|---|---|---|---|
| Signal word: | ||||||||
| Hazard statements: |
|
|||||||
| Precautionary statements: |
|
Description
More related product prices
(2S,5R)-2,5-DIMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER (2S,5S)-2,5-DIMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER (2R,5S)-2,5-DIMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER (2R,3S)-3-(tert-Butoxycarbonylamino)-1-chloro-2-hydroxy-4-phenylbutane (2R,5R)-1,6-Diphenylhexane-2,5-diaMine dihydrochloride (2R)-4-(PhenylMethyl)-2-MorpholineMethanaMine




