| Identification | Back Directory | [Name]
5-chloro-3-hydroxyisothiazole | [CAS]
25629-58-7 | [Synonyms]
5-Chloro-3-isothiazolol
5-chloroisothiazol-3-ol
5-Chloro-3(2H)-isothiazolone
5-chloro-3-hydroxyisothiazole
5-Chloro-4-isothiazolin-3-one
3(2H)-Isothiazolone, 5-chloro-
5-chloro-3(2H)-Isothiazolone 97% | [Molecular Formula]
C3H2ClNOS | [MDL Number]
MFCD16658894 | [MOL File]
25629-58-7.mol | [Molecular Weight]
135.57 |
| Chemical Properties | Back Directory | [Melting point ]
96-97 °C(Solv: water (7732-18-5)) | [density ]
1.62±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [pka]
7.20±0.40(Predicted) |
| Hazard Information | Back Directory | [Synthesis]
5-chloro-3-hydroxyisothiazole can be synthesized from 3,3'-disulfanediyldipropionic acid and Thionyl chloride in three steps. In particular, in the last step, 5-chloro isothiazolone derivatives (5-chloro-3-hydroxyisothiazole) were the predominant products when the ratio of sulfuryl chloride/amide was 3:1 while with the relevant ratio of 1:1, the 5-unsubstituted analogs were the predominant products[1].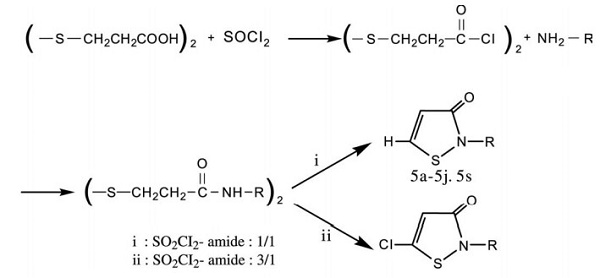 | [References]
[1] Khalaj A, et al. Synthesis and antibacterial activity
of 2-(4-substituted phenyl)-3(2H)-isothiazolones. European Journal of Medicinal Chemistry, 2004; 39: 285–290.
|
|
|