| Identification | Back Directory | [Name]
Vincristine sulfate | [CAS]
2068-78-2 | [Synonyms]
VCR
oncovin
onkovin
nsc67574
Vincrisul
NovopharM
vincristin
V5 PEPTIDE
lilly37231
kyocristine
VCR SULFATE
Vincasar PFS
LEUROCRISTINE
vinorelbine�����,NVB
VINCRISTINE, VCR
vincristinsulfat
Vincristin Sulfate
VINCRISTINE SULFATE
VINCRISTINE SULPHATE
LEUROCRISTINE SULFATE
LEUROCRISTINESULPHATE
Vincristine sulfate sa
Vincristine sulfate,95%
VincristineSulphateBp93
VINCRISTINE SULFATE(RG)
VINCRISTINE SULFATE USP
22-OXOVINCALEUKOBLASTINE
VINCRISTINE SULFATE SALT
22-Oxovinacleukoblastine
Vincristine Sulphate USP
Vinecristine sulfate salt
leurocristinesulfate(1:1)
LEUROCRISTINE SULFATE SALT
VCR, LEUKOCRISTINE SULFATE
Vincristine sulphate USP25
Vincristine, sulphate salt
Vincristine sulfate (assay)
Vincristine Sulfate (50 mg)
Leurocristine sulfate (7CI)
Vincristine sulfate(NSC 67574)
VincristineSulphateUsp28/Bp2003
leurocristinesulfate(1:1)(salt)
VINCRISTINE SULFATE, EP STANDARD
VINCRISTINE SULFATE, USP 95-101%
22-Oxovinacleukoblastine sulfate
VINCRISTINE SULFATE, USP STANDARD
22-OXOVINCALEUKO-BLASTINE SULFATE
VINCRISTINE SULFATE, WHO STANDARD
Vincristine Sulfate (50 mg/ampule)
Vincristine Sulfate, Apocynaceae sp.
22-OXOVINCALEUKOBLASTINE SULFATE SALT
VCR, LCR, Kyocristine, Oncovin, Vincrex
Vincristine sulfate, froM Vinca rosea L.
22-oxo-vincaleukoblastine, sulfate (1:1)
VINCRISTINE SULFATE, APOCYNACEAE SPECIES
Leurocristine, sulfate (1:1) (salt) (8CI)
22-oxo-vincaleukoblastinsulfate(1:1)(salt)
Vincristine Sulfate (Assay) (29.8 mg/vial)
Vincaleukoblastine, 22-oxo-, sulfate (1:1)
Vincristine���,Leurocristine,Oncovin��,Vinblastine
Vincaleukoblastine, 22-oxo-, sulfate (1:1) (salt)
VINCRISTINE SULFATE, REFERENCE SPECTRUM EP STANDARD
Vincaleukoblastine, 22-oxo-, sulfate (1:1) (salt) (9CI)
Vincristine sulfate
22-Oxovincaleukoblastine sulfate salt
22-Oxovincaleukoblastine sulfate salt
Leurocristine sulfate salt
VCR
22-Oxovincaleukoblastine sulphate salt, Leurocristine sulphate salt, VCR
Vincristine sulfate salt,22-Oxovincaleukoblastine sulfate salt, Leurocristine sulfate salt, VCR | [EINECS(EC#)]
218-190-0 | [Molecular Formula]
C46H58N4O14S | [MDL Number]
MFCD00084729 | [MOL File]
2068-78-2.mol | [Molecular Weight]
923.04 |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Solid | [Melting point ]
300 °C
| [alpha ]
D26 +8.5° (c = 0.8) | [Boiling point ]
273-281 °C
| [storage temp. ]
2-8°C
| [solubility ]
methanol: soluble20mg/mL | [form ]
lyophilized powder
| [color ]
white to off-white | [Water Solubility ]
>=1 g/100 mL at 24 ºC | [BRN ]
3924631 | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 3 months. | [InChIKey]
AQTQHPDCURKLKT-HSMPTBRCNA-N | [IARC]
3 (Vol. 26, Sup 7) 1987 | [EPA Substance Registry System]
Vincaleukoblastine, 22-oxo-, sulfate (1:1) (salt)(2068-78-2) |
| Hazard Information | Back Directory | [Chemical Properties]
Crystalline Solid | [Uses]
An antitumor alkaloid isolated from Vinca rosea Linn. An antineoplastic. | [Uses]
H1-antihistamine | [General Description]
An anticancer drug. White to slightly yellow, amorphous or crystalline powder. Sensitive to light. Odorless. pH (0.1% solution) 3.5 - 4.5. | [Air & Water Reactions]
Very hygroscopic. Water soluble. | [Reactivity Profile]
Sensitive to hydrolysis, oxidation and heat. Incompatible with strong oxidizing agents. . | [Fire Hazard]
Flash point data for Vincristine sulfate are not available; however, Vincristine sulfate is probably combustible. | [Biological Activity]
Anticancer agent; microtubule disrupter. Induces apoptosis in human lymphoma cells. | [Description]
Vincristine sulfate (2068-78-2) arrests cell cycle at G2/M by interfering with mitotic spindle formation. Depolymerizes microtubules and blocks binding of tubulin to microtubule proteins.1,2?Induces apoptosis.3?Vincristine sulfate is a clinically useful cancer chemotherapeutic agent. | [Originator]
Oncovin,Lilly ,US ,1963 | [Manufacturing Process]
The alkaloid mixture from the extraction of Vinca rosea plants (as in
vinblastine extraction) was chromatographed to give vincristine which was
then converted to the sulfate, according to US Patent 3,205,220.
Vincristine may also be prepared in a semisynthetic process starting from
vinblastine. Vinblastine or a salt thereof, preferably the sulfate, is oxidized
with chromic acid or with one of its salts at a low temperature, the reaction
mixture is neutralized or rendered alkaline and the product is separated
therefrom by extraction, the extract is evaporated to dryness, the dry residue
is optionally formylated, vincristine, and optionally N-demethylvinblastine also,
are isolated from the product, and the product(s) are optionally converted into
their salts; preferably into the sulfates, according to US Patent 3,899,493. | [Brand name]
Oncovin (Lilly); Vincasar (Sicor). | [Therapeutic Function]
Cancer chemotherapy | [Clinical Use]
Antineoplastic agent | [Veterinary Drugs and Treatments]
Vincristine is used as an antineoplastic primarily in combination
drug protocols in dogs and cats in the treatment of lymphoid and
hematopoietic neoplasms. In dogs, it may be used alone in the therapy
of transmissible venereal neoplasms.
Because vincristine can induce thrombocytosis (at low doses)
and has some immunosuppressant activity, it may also be employed
in the treatment of immune-mediated thrombocytopenia. | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: possible increased risk of ventricular
arrhythmias with delamanid.
Antiepileptics: phenytoin levels may be reduced.
Antifungals: metabolism possibly inhibited by
itraconazole and posaconazole (increased risk of
neurotoxicity).
Antimalarials: avoid with piperaquine with
artenimol.
Antipsychotics: avoid concomitant use with
clozapine (increased risk of agranulocytosis).
Cytotoxics: toxicity possibly increased by
asparaginase, crisantaspase and pegasparagase
- give at least 3-24 hours before asparaginase,
crisantaspase and pegasparagase; increased risk of
hepatotoxicity with dactinomycin. | [Metabolism]
Vincristine is metabolised in the liver by the cytochrome P450 isoenzymes CYP3A4 and CYP3A5 and excreted mainly in the bile; about 70-80
% of a dose is found in faeces, as unchanged drug and metabolites (40-50
%), while 10-20
% appears in the urine. | [storage]
Store at -20°C | [Purification Methods]
The salt is recrystallised from MeOH. It has UV max at 220, 255 and 296nm (log � 4.65, 4.21 and 4.18). It is a monoamine oxidase inhibitor and is used in cancer research [Son et al. J Med Chem 33 1845 1990, Horio et al. Proc Natl Acad Sci USA 85 3580 1988]. | [Mode of action]
Vincristine Sulfate is the sulfate salt of a natural alkaloid isolated from the plant Catharanthus roseus (Vinca rosea L.) with antimitotic and antineoplastic activities. Vincristine binds irreversibly to microtubules and spindle proteins in S phase of the cell cycle and interferes with the formation of the mitotic spindle, thereby arresting tumor cells in metaphase. This agent also depolymerizes microtubules and may also interfere with amino acid, cyclic AMP, and glutathione metabolism; calmodulin-dependent Ca(2+)-activated ATPase activity; cellular respiration; and nucleic acid and lipid biosynthesis. | [Toxicity evaluation]
The mostcommonly seen toxicity for vincristine is a dose-limitingneurotoxicity caused by effects on axonal microtubules.Symptoms are variable and include peripheral neuropathy,ataxia, seizure, bone pain, and coma. Constipation is also acommonly seen toxicity, and laxatives may be used prophylactically.Other toxicities include alopecia, skin rash, mildmyelosuppression, secretion of antidiuretic hormone,azospermia, and amenorrhea. | [References]
1) Jordan et al. (1998), Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle; Med. Res. Rev., 18 259
2) Lobert et al. (1996), Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinorelbine; Biochemistry, 35 6806
3) Wang et al. (1999), The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review: Cancer Chemother. Pharmacol., 44 355 |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
61-36/37/38-63-23/24/25-68-62-25 | [Safety Statements ]
22-24/25-53-45-37/39-26-36/37/39-36/37 | [RIDADR ]
UN 2811 6.1/PG 2
| [WGK Germany ]
3
| [RTECS ]
OH6340000
| [F ]
8-10 | [HazardClass ]
6.1(a) | [PackingGroup ]
II | [HS Code ]
29399990 | [Reaction]
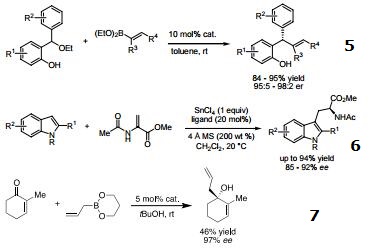
- Ligand used to prepare a chiral zirconium catalyst useful in asymmetric Strecker reactions.
- Ligand used in the zinc-catalyzed enantioselective Hetero Diels-Alder reaction.
- Catalyst used in syn-selective diastereoselective Petasis reactions.
- Ligand for zirconium(IV) and hafnium(IV)-catalyzed asymmetric Friedel-Crafts alkylation of indoles.
- Catalyst used in asymmetric aryl- and alkenylboration of o-quinone methides.
- Ligand used in the tandem indole Friedel-Crafts conjugate addition/asymmetric protonation reaction.
- Catalyst in asymmetric conjugate allylation of cyclic enones.

| [Toxicity]
LD50 intraperitoneal in mouse: 3mg/kg |
|
|