| Identification | Back Directory | [Name]
AP20187 | [CAS]
195514-80-8 | [Synonyms]
AP20187
B/B Homodimerizer
AP20187
(AP-20187
AP20187;AP-20187;AP 20187
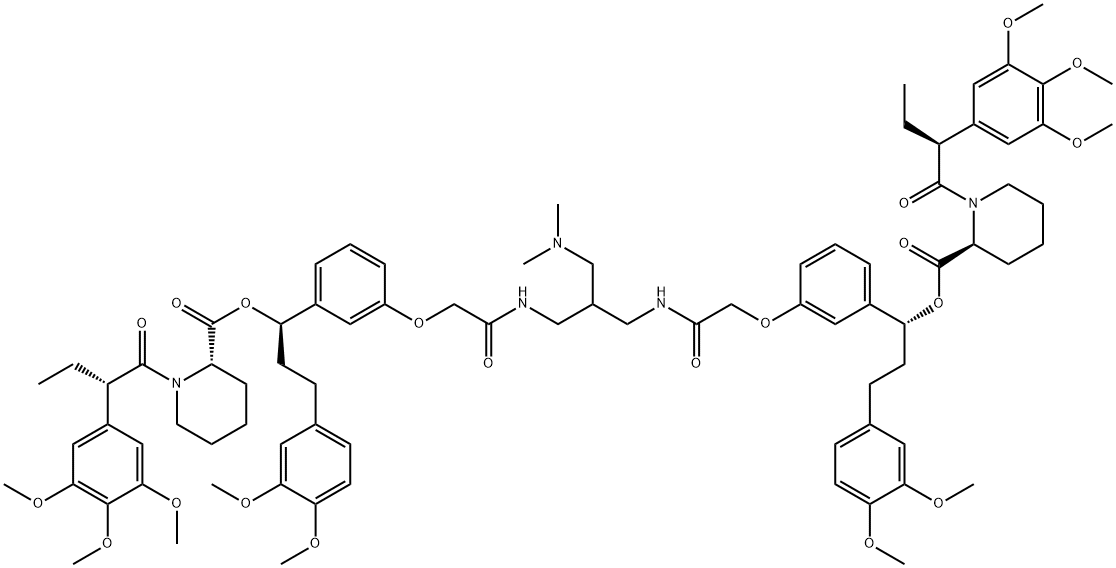
(1R,1'R)-(((((2-((dimethylamino)methyl)propane-1,3-diyl)bis(azanediyl))bis(2-oxoethane-2,1-diyl))bis(oxy))bis(3,1-phenylene))bis(3-(3,4-dimethoxyphenyl)propane-1,1-diyl) (2S,2'S)-bis(1-((S)-2-(3,4,5-trimethoxyphenyl)butanoyl)piperidine-2-carboxylate)
(1R)-3-(3,4-dimethoxyphenyl)-1-[3-[2-[[2-[[[2-[3-[(1R)-3-(3,4-dimethoxyphenyl)-1-[(2S)-1-[(2S)-2-(3,4,5-trimethoxyphenyl)butanoyl]piperidine-2-carbonyl]oxypropyl]phenoxy]acetyl]amino]methyl]-3-(dimethylamino)propyl]amino]-2-oxoethoxy]phenyl]propyl] (2S)-1-[(2S)-2-(3,4,5-trimethoxyphenyl)butanoyl]piperidine-2-carboxylate | [Molecular Formula]
C82H107N5O20 | [MDL Number]
MFCD28167729 | [MOL File]
195514-80-8.mol | [Molecular Weight]
1482.75 |
| Chemical Properties | Back Directory | [Boiling point ]
1333.5±65.0 °C(Predicted) | [density ]
1.192±0.06 g/cm3(Predicted) | [storage temp. ]
Store at -20°C,protect from light | [solubility ]
≥74.14 mg/mL in DMSO; ≥100 mg/mL in EtOH | [form ]
A solid | [pka]
13.97±0.46(Predicted) | [color ]
White to yellow |
| Hazard Information | Back Directory | [Definition]
ChEBI: AP20187 is a tertiary amino compound that is 2-(aminomethyl)-N,N-dimethylpropane-1,3-diamine in which the primary ammino groups have each been acylated by condensation with the carboxy group of 2-{3-[(1R)-3-(3,4-dimethoxyphenyl)-1-hydroxypropyl]phenoxy}acetic acid, the hydroxy groups of which have been esterified by condensation with the carboxy group of L-pipecolic acid, the nitrogen of which has been acylated by condensation with (2S)-2-(3,4,5-trimethoxyphenyl)butyric acid. It is a synthetic, cell-permeable ligand that can be used to induce homodimerization of fusion proteins containing the DmrB domain. It has a role as a ligand. It is a N-acylpiperidine, a carboxylic ester, an aromatic ether and a tertiary amino compound. | [Biological Activity]
ap20187 is a small dimerizer drug [1].to solve the graft-versus-host disease, the in vivo behavior of the transplanted cells should be controlled. ap20187 is used in the conditional system as a chemical inducer of dimerization (cid). another component is a fusion protein. the cids have advantages in gene therapy, it offers the possibility of achieving selection without the toxic effects. in vivo studies show that ap20187 can produces a notable expansion of transduced red cells, platelets, and to a lesser extent, granulocytes [1].ap20187 is also reported to be used in an ap20187–lfv2ire system. in this system, ap20187 administration causes the activation of lfv2ire and results in increased uptake of both hepatic glycogen content and muscular glucose [2]. | [in vitro]
When LNCaP cells are treated with AP20187 (B/B Homodimerizer) (100 nM), ro-iCaspase-9 levels are significantly reduced, and the smaller processed active caspase-9 becomes apparent. < p> | [in vivo]
Real-time PCR analysis showed that AP20187 (B/B Homodimerizer) (0.5 mg/kg, 2 mg/kg, or 5 mg/kg) treatment significantly increased the levels of CHOP mRNA in the CNS of < i> PLP/Fv2E-PERK mice at PID12. AP20187 treatment significantly alleviates EAE-induced myelin damage in these mice. AP20187 (B/B Homodimerizer) treatment significantly reduces the number of degenerating axons and increases the density of axons in the demyelinating lesions in the lumbar spinal cord of PLP/Fv2E-PERK mice. | [target]
| [storage]
Store at -20°C | [References]
[1] neff t, blau ca. pharmacologically regulated cell therapy. blood. 2001 may 1;97(9):2535-40.
[2] cotugno g, formisano p, giacco f, colella p, beguinot f, auricchio a. ap20187-mediated activation of a chimeric insulin receptor results in insulin-like actions in skeletal muscle and liver of diabetic mice. hum gene ther. 2007 feb;18(2):106-17. |
|
|