| Identification | Back Directory | [Name]
1,8-DIBROMONAPHTALENE | [CAS]
17135-74-9 | [Synonyms]
CASC
1,8-DBN
1,8-dibromonalene
8-DIBROMONAPHTALENE
1,8-DibromonaphthaL
1,8-dibroMophthalene
1,8-DIBROMONAPHTALENE
1,8-dibromonaphthylene
Naphthalene,1,8-dibromo-
1,8-Dibromonaphthalene >
1,8-dibroMonaphthalene
1,8-DBN
Ethanol,2-[2-(5-piperazinyl)ethoxy]-
1,8-DIBROMONAPHTALENE ISO 9001:2015 REACH | [Molecular Formula]
C10H6Br2 | [MDL Number]
MFCD00183574 | [MOL File]
17135-74-9.mol | [Molecular Weight]
285.96 |
| Chemical Properties | Back Directory | [Melting point ]
108.0 to 112.0 °C | [Boiling point ]
140°C/0.1mmHg(lit.) | [density ]
1.834±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
soluble in Toluene | [form ]
powder or crystals | [color ]
White to Yellow to Green | [InChIKey]
DLXBGTIGAIESIG-UHFFFAOYSA-N |
| Hazard Information | Back Directory | [Chemical Properties]
White to yellow powder/crystals. | [Application]
1,8-Diarylnaphthalene show great potential in highly efficient photoluminescent blue and green OLEDs, chiral ligands and sensors, nonlinear optic chromophores, stereo-dynamic switches and other optoelectronic devices.
| [Preparation]
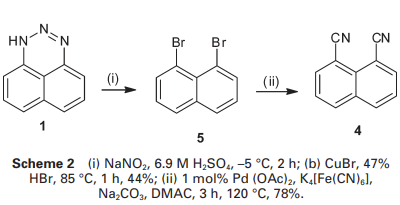
1,8-dibromonaphthalene was prepared by diazotisation of triazine 1 with sodium nitrite in sulfuric acid, followed by the addition of CuBr/HBr in 44% yield, and cyanation of the dibromide (5) by Weissman’s method gave naphthalene-1,8-dicarbonitrile (4) in 78% yield (Scheme 2).
 | [Reactions]
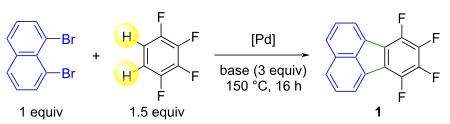
Influence of the conditions on the Pd-catalyzed reaction of 1,8-dibromonaphthalene with 1,2,3,4-tetrafluorobenzene.
Scope of the Pd-catalyzed direct arylation reaction of 2,5-substituted heteroarenes with 1,8-dibromonaphthalene.
 | [Structure and conformation]
1,8-Dibromonaphthalene (1,8-DBN) is a symmetrical double bromo-substituted naphthalene with two bromos sitting at the naphthalene ring mirroring each other along the joining carbons.
| [References]
[1] W. NOLAND D. B Venkata Srinivasarao Narina. Synthesis and Crystallography of 8-Halonaphthalene-1-Carbonitriles and Naphthalene-1,8-Dicarbonitrile[J]. Journal of Chemical Research-s, 2011, 383 1: 694-697. DOI:10.3184/174751911X13222107572093.
[2] PROF. DR. ANNA V. GULEVSKAYA Eugeny A E. 1,8‐Diarylnaphthalenes: Synthesis, Properties, and Applications[J]. European Journal of Organic Chemistry, 2022, 2022 48: Pages 53-111. DOI:10.1002/ejoc.202201192.
[3] KETATA N, LIU L, SALEM R B, et al. Mono or double Pd-catalyzed C–H bond functionalization for the annulative π-extension of 1,8-dibromonaphthalene: a one pot access to fluoranthene derivatives[J]. Beilstein Journal of Organic Chemistry, 2024, 16 1. DOI:10.3762/bjoc.20.37. |
|
|