| Identification | Back Directory | [Name]
(R)-(+)-2-[2-(DIPHENYLPHOSPHINO)PHENYL]-4-(1-METHYLETHYL)-4,5-DIHYDROOXAZOLE | [CAS]
164858-78-0 | [Synonyms]
(R)-(+)-2-[2-(Diphenylphosphino
(R)-(+)-2-[2-(Diphenylphosphino)phenyl]-4-isopropyl-2-oxazol
(R)-(+)-2-[2-(DIPHENYLPHOSPHINO)PHENYL]-4-ISOPROPYL-2-OXAZOLINE
(R)-2-(2-(Diphenylphosphino)phenyl)-4-isopropyl-4,5-dihydrooxazole
(R)-(+)-2-[2-(DIPHENYLPHOSPHINO)PHENYL]-4,5-DIHYDRO-4-ISOPROPYL-OXAZOLE
(R)-(+)-2-[2-(Diphenylphosphino)Phenyl]-4-(1-Methyl)-4,5-Dihydrooxazole
(4R)-(+)-4,5-DIHYDRO-2-(2'-(DIPHENYLPHOSPHINO)PHENYL)-4-ISOPROPYLOXAZOLE
(R)-(+)-2-[2-(DIPHENYLPHOSPHINO)PHENYL]-4-(1-ISOPROPYL)-4,5-DIHYDROOXAZOLE
(4R)-2-[2-(diphenylphosphino)phenyl]-4,5-dihydro-4-(1-Methylethyl)-Oxazole
(R)-(+)-2-[2-(DIPHENYLPHOSPHINO)PHENYL]-4-(1-METHYLETHYL)-4,5-DIHYDROOXAZOLE
(4R)-(+)-4,5-Dihydro-2-[2'-(diphenylphosphino)phenyl]-4-isopropyloxazole,98%
(R)-(+)-2-[2-(Diphenylphosphino)phenyl]-4-isopropyl-2-oxazoline >=97.0% (CHN)
(R)-(+)-2-[2-(Diphenylphosphino)phenyl]-4-(1-methylethyl)-4,5-dihydrooxazole,98%
(4R)-2-[2-(Diphenylphosphino)phenyl]-4,5-dihydro-4-(1-methylethyl)oxazole,99%e.e. | [Molecular Formula]
C24H24NOP | [MDL Number]
MFCD02684553 | [MOL File]
164858-78-0.mol | [Molecular Weight]
373.43 |
| Chemical Properties | Back Directory | [Appearance]
White crystalline powder | [Melting point ]
85-90 °C
| [alpha ]
+44° (c 1.4, CHCl3) | [Boiling point ]
492.7±28.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [form ]
Powder | [pka]
4.31±0.70(Predicted) | [color ]
white | [optical activity]
[α]20/D +48±3°, c = 1.4% in chloroform | [Sensitive ]
air sensitive | [BRN ]
8156604 | [InChIKey]
OUQSAXROROGQEE-QFIPXVFZSA-N | [CAS DataBase Reference]
164858-78-0 |
| Questions And Answer | Back Directory | [Reaction]
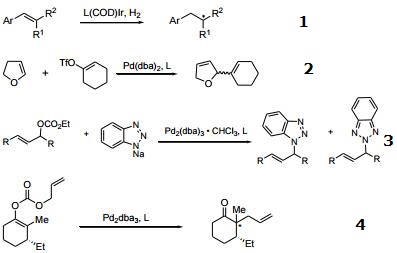
Chiral ligand used in the asymmetric reduction of highly substituted olefins.
Chiral ligand used in the enantioselective Heck reaction. The success of the reaction is due to the fact that the catalytic system does not promote double bond isomerization.
Chiral ligand used in the entantioselective palladium-catalyzed allylic substitution of sodium benzotriazole.
Decarboxylative allylic alkylation.
|
|
|