| Identification | Back Directory | [Name]
t-boc-N-amido-PEG2-bromide | [CAS]
164332-88-1 | [Synonyms]
CAS_164332-88-1
N-Boc-PEG1-bromide
t-boc-N-amido-PEG2-bromid
t-boc-N-amido-PEG2-bromide
t-boc-N-amido-PEG1-bromide
tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate
tert-Butyl N-[2-(2-bromoethoxy)ethyl]carbamate
Carbamic acid, N-[2-(2-bromoethoxy)ethyl]-, 1,1-dimethylethyl ester | [Molecular Formula]
C9H18BrNO3 | [MDL Number]
MFCD24167474 | [MOL File]
164332-88-1.mol | [Molecular Weight]
268.15 |
| Chemical Properties | Back Directory | [Boiling point ]
336.6±22.0 °C(Predicted) | [density ]
1.283±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Store in freezer, under -20°C | [solubility ]
Soluble in Water, DMSO, DCM, DMF | [form ]
Liquid | [pka]
12.19±0.46(Predicted) | [color ]
Colorless to light yellow |
| Hazard Information | Back Directory | [Description]
t-boc-N-amido-PEG2-bromide is a PEG derivative containing a bromide group and Boc-protected amino group. The hydrophilic PEG spacer increases solubility in aqueous media. The bromide (Br) is a very good leaving group for nucleophilic substitution reactions. The Boc group can be deprotected under mild acidic conditions to form the free amine. | [Synthesis]
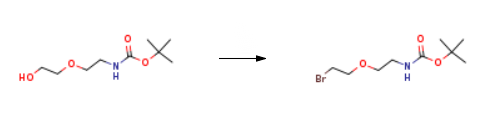
The synthesis of t-boc-N-amido-PEG2-bromide is as follows:
Alcohol 64 (1 .25 g, 6.09 mmol) was dissolved in DCM (34 mL). The solution was cooled to 0°C and methanesulfonyl chloride (MsCI, 0.80 mL, 10.3 mmol, 1 .7 equiv) was added to the solution followed by Et3N (1.9 mL, 13.4 mmol, 2.2 equiv). After stirring for 3 h at rt, the reaction mixture was diluted with acetone (33 mL) and LiBr (8.9 g, 103 mmol, 17 equiv) was added. The reaction mixture was stirred overnight at rt. After that time, the solvents were evaporated under reduced pressure. The crude residue was diluted with EtOAc and washed with H20 and brine. The organic phase was dried over anhyd Na2S04. The suspension was filtered over cotton and the filtrate concentrated in vacuo. Purification by flash chromatography eluting with PE/Acetone (85:15→ 8:2) gave bromide 65 (1 .60 g, 5.95 mmol, 98%) as a colourless oil.
 | [Uses]
tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate is a cleavable ADC linker for the synthesis of antibody drug conjugates (ADCs). | [Biological Activity]
The chemical structure of tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate consists of a tert-butyl carbamate group attached to a N-methylcarbamate moiety via a 2-(2-bromoethoxy)ethyl linker. This unique structure contributes to its potential biological activity. The compound has been found to exhibit inhibitory effects on specific biological targets, primarily through its mechanism of action involving the disruption of cell function and signal transduction pathways. The potency of tert-butyl N-[2-(2-bromoethoxy)ethyl]-N-methylcarbamate in modulating these targets varies depending on the specific biological system under investigation. | [Biotechnological Applications]
The biological effects of tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate on cell function and signal transduction have been extensively studied. It could interfere with various cellular processes, including cell proliferation, apoptosis, and differentiation. Additionally, the compound has demonstrated potential therapeutic effects in certain disease models, highlighting its potential as a drug candidate. However, it is important to consider the potential toxic effects of tert-butyl N-[2-(2-bromoethoxy)ethyl]-N-methylcarbamate, as excessive exposure or misuse may lead to adverse health outcomes. |
|
|