| Identification | Back Directory | [Name]
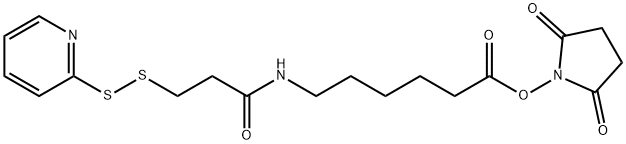
Succinimidyl 6-[3-(2-Pyridyldithio)propionamido]hexanoate | [CAS]
158913-22-5 | [Synonyms]
SPDP-C6-NHS ester
N-succinimidyl 6-(3-(2-pyridyldithio)propionamido)hexanoate
2,5-dioxopyrrolidin-1-yl 6-(3-(pyridin-2-yldisulfaneyl)propanamido)hexanoate
N-[6-[(2,5-Dioxo-1-pyrrolidinyl)oxy]-6-oxohexyl]-3-(2-pyridinyldithio)propanaMide
Hexanoic acid, 6-[[1-oxo-3-(2-pyridinyldithio)propyl]amino]-, 2,5-dioxo-1-pyrrolidinyl ester | [Molecular Formula]
C18H23N3O5S2 | [MDL Number]
MFCD00083163 | [MOL File]
158913-22-5.mol | [Molecular Weight]
425.52 |
| Hazard Information | Back Directory | [Description]
SPDP-C6-NHS ester contains a disulfide bond which is cleavable upon reduction. The NHS ester is reactive toward primary amine and sulfhydryl groups. SPDP-C6-NHS ester is commonly used for bioconjugation and reversible crosslinkings. | [Chemical Properties]
White Solid | [Uses]
A sulfhydryl and amino reactive heterobifunctional protein crosslinking reagent | [Uses]
A sulfhydryl and amino reactive heterobifunctional protein crosslinking reagent. | [General Description]
The amine-reactive portion of SPDP reagents is the N-hydroxysuccinimide (NHS) ester. Reactions are most commonly performed in phosphate, carbonate/bicarbonate, or borate buffers at pH 7-8. Other buffers can be used provided they do not contain primary amines (or thiols or disulfide reducing reagents – see discussion of the sulfhydryl reactivity). The rate of reaction and degradation by hydrolysis increases with increasing pH; for example, the half-life of the NHS ester is several hours at pH 7 and less than 10 minutes at pH 9. NHS-ester reagents like SPDP and LC-SPDP have limited aqueous solubility and must be dissolved in organic solvent before adding them to a reaction mixture. Sulfo-NHS-ester reagents like Sulfo-LC-SPDP are water-soluble and can be added directly to aqueous reaction mixtures. |
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|