| Identification | Back Directory | [Name]
levallorphan | [CAS]
152-02-3 | [Synonyms]
C07069
MCL 113
Naloxiphan
Levalorfano
levallorphan
Levallorphane
Levallorphanum
17-Allylmorphinan-3-ol
N-Allyl-3-hydroxymorphinan
l-N-Allyl-3-hydroxymorphinan
17-(2-propenyl)morphinan-3-ol
(-)-3-Hydroxy-N-allylmorphinan
Morphinan-3-ol, 17-allyl- (8CI)
Morphinan-3-ol, 17-(2-propen-1-yl)-
Morphinan-3-ol, 17-(2-propenyl)- (9CI)
2H-10,4a-Iminoethanophenanthren-6-ol, 11-allyl-1,3,4,9,10,10a-hexahydro- (6CI) | [EINECS(EC#)]
205-799-1 | [Molecular Formula]
C19H25NO | [MDL Number]
MFCD00867732 | [MOL File]
152-02-3.mol | [Molecular Weight]
283.41 |
| Chemical Properties | Back Directory | [Melting point ]
180-182° | [alpha ]
D20 -88.9° (c = 3 in methanol) | [Boiling point ]
425.96°C (rough estimate) | [density ]
1.0260 (rough estimate) | [refractive index ]
1.5000 (estimate) | [pka]
10.17±0.20(Predicted) |
| Hazard Information | Back Directory | [Definition]
ChEBI: Levallorphan is a morphinane alkaloid. | [Brand name]
Lorfan (Roche). | [Clinical Use]
Levallorphan
is an opioid antagonist with practically
no analgesic action. It has been used as
one of the first relative pure antagonists for the
treatment of opioid overdosage, to reverse opioid
central depression and to antagonize opioidinduced
respiratory impairment . The compound
is now replaced by naloxone . | [Synthesis]
Starting material for the levallorphan
synthesis is 1-(4-methoxybenzyl)-
1,2,3,4,5,6,7,8-octahydro isoquinoline (1)
.
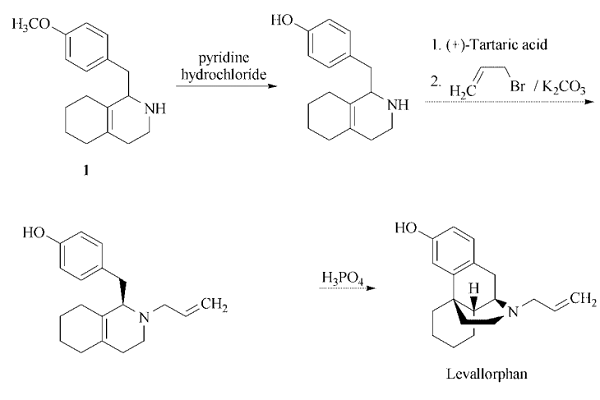 | [Purification Methods]
It crystallises from aqueous EtOH. It is a narcotic antagonist. [Schneider & Grüssner Helv Chim Acta 34 2211 1951, Hellerbach Helv Chim Acta 39 429 1956.] |
|
| Company Name: |
CHEMICAL LAND21
|
| Tel: |
82- 2 -783 - 8063 |
| Website: |
www.chemicalland21.com |
|