| Identification | Back Directory | [Name]
Tolvaptan | [CAS]
150683-30-0 | [Synonyms]
SaMsca
Tolvptan
TOIVAPTAN
Tolvaptan
OPC 41061
OPC-41061
Tolvaptan-d7
Tolvaptan Tablets
pazopanib hydrochloeide
N-(4-(7-Chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-benzo[b]-azepine-1-carbonyl)-3-methylphenyl)-2-
N-(4-(7-Chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-benzo[b]-azepine-1-carbonyl)-3-methylphenyl)-2-met
N-[4-(7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1-benzazepine-1-carbonyl)-3-methylphenyl]-2-methylbenzamide
N-(4-(7-Chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-benzo[b]azepine-1-carbonyl)-3-Methylphenyl)-2-MethylbenzaMide
N-[4-[(5R)-7-Chloro-5-hydroxy-2,3,4,5-tetrahydro-1-benzazepine-1-carbonyl]-3-methylphenyl]-2-methylbenzamide
N-[4-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepin-1-yl)carbonyl]-3-methylphenyl]-2-methyl-benzamide
(R)-N-(4-(7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-benzo[b]azepine-1-carbonyl)-3-methylphenyl)-2-methylbenzamide
N-[4-(9-chloro-6-hydroxy-2-azabicyclo[5.4.0]undeca-8,10,12-triene-2-carbonyl)-3-methyl-phenyl]-2-methyl-benzamide
N-(4-{[(5R)-7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]carbonyl}-3-Methylphenyl)-2-MethylbenzaMide | [EINECS(EC#)]
691-537-5 | [Molecular Formula]
C26H25ClN2O3 | [MDL Number]
MFCD09838782 | [MOL File]
150683-30-0.mol | [Molecular Weight]
448.94 |
| Chemical Properties | Back Directory | [Melting point ]
219-222°C | [Boiling point ]
594.4±50.0 °C(Predicted) | [density ]
1.311±0.06 g/cm3(Predicted) | [storage temp. ]
Refrigerator | [solubility ]
DMSO: ≥15mg/mL | [form ]
powder | [pka]
13.00±0.70(Predicted) | [color ]
white to tan | [InChIKey]
GYHCTFXIZSNGJT-UHFFFAOYSA-N | [SMILES]
C(NC1=CC=C(C(N2C3=CC=C(Cl)C=C3C(O)CCC2)=O)C(C)=C1)(=O)C1=CC=CC=C1C |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Uses]
It is a selective, competitive arginine vasopressin V2 receptor antagonist
f inappropriate antidiuretic hormone (SIADH). | [Uses]
Labelled Tolvaptan
s, and the syndrome of inappropriate antidiuretic hormone (SIADH). | [Uses]
Tolvaptan (OPC-41061) is a selective, competitive arginine vasopressin receptor 2 antagonist with an IC50 of 1.28μM for the inhibition of AVP-induced platelet aggregation. Tolvaptan (OPC-41061) is used to treat hyponatremia (low blood sodium levels) assoc | [Originator]
Otsuka Pharmaceutical (US) | [Definition]
ChEBI: Tolvaptan is a benzazepine derivative incorporating a benzenedicarboxamide function whch is a selective vasopressin V2 receptor antagonist used to treat euvolemic and hypervolemic hyponatremia. It is also used in the treatment of rapidly progressing autosomal dominant polycystic kidney disease to slow the rate of cyst development and renal insufficiency. Tolvaptan is a racemate consisting of a 1:1 mixture of 5R and 5S stereomers. It has a role as a vasopressin receptor antagonist. It is a benzazepine and a benzenedicarboxamide. | [Brand name]
Samsca | [Biochem/physiol Actions]
Tolvaptan (OPC 41061) is a potent, orally active non-peptide vasopressin V2 selective antagonist. IC50 = 3 nM at the rat V2 receptor; 29 times more selective for the V2 than for V1a. Tolvaptan has also been shown to inhibit the development of polycystic kidney disease in several animal models. | [Clinical Use]
Tolvaptan, also known as OPC-41061, is a selective, competitive
arginine vasopressin receptor 2 antagonist used to treat hyponatremia
(low blood sodium levels) associated with congestive heart
failure, cirrhosis, and the syndrome of inappropriate antidiuretic
hormone (SIADH). Otsuka Pharmaceutical licensed tolvaptan
under the trade name Samsca after the FDA approved the drug in
May 2009. Tolvaptan has also shown efficacy against polycystic
kidney disease. In a 2004 trial, tolvaptan administered with traditional
diuretics was noted to increase excretion of excess fluids and
improve blood sodium levels in patients with heart failure without
producing side effects such as hypotension (low blood pressure) or
hypokalemia (decreased blood levels of potassium). The drug also
exhibited no adverse effect on kidney function. | [Side effects]
The most common adverse events were thirst, dry mouth, asthenia, constipation, pollakiuria or polyuria, and hyperglycemia. The recommended starting dose is 15 mg daily with a daily 15-mg adjustment to a maximum of 60 mg daily to raise serum sodium concentration. Initiation should be in a hospital setting where serum sodium and volume status may be monitored since too rapid correction of hyponatremia (>12 mEq/L/24 h) can cause osmotic demyelination resulting in dysarthria, mutism, dysphagia, lethargy, spastic quadriparesis, seizures, coma, and death. In addition to avoiding concomitant use of strong CYP3A4 inhibitors, tolvaptan is contraindicated in settings of urgent need to raise serum sodium acutely, in patients with an inability to sense or appropriately respond to thirst, in hypovolemic hyponatremia conditions, and in anuric patients. | [Synthesis]
Tolvaptan may be prepared in 11 steps starting from 5chloro- 2-nitrobenzoic acid. Following esterification, reduction of the nitro moiety with tin(II) chloride, subsequent protection (tosylation) and alkylation of the resulting aniline, and a Dieckmann cyclization with potassium tert-butoxide generate the benzazepinone core that is ultimately decarboxylated by heating with hydrochloric acid in acetic acid. After deprotection of the amine group, condensation with 2methyl- 4-nitrobenzoyl chloride affords another nitro handle that is reduced with tin(II) chloride. This aniline is coupled with 2-methylbenzoyl chloride to give the penultimate intermediate. In the final step, reduction of the ketone functionality with sodium borohydride provides racemic tolvaptan that is formulated into 15- and 30-mg tablets for oral administration. | [storage]
Store at +4°C |
| Questions And Answer | Back Directory | [Description]
Tolvaptan(trade names Samsca and Jinarc) is an oral selective vasopressin antagonist developed by Otsuka for the treatment of hyponatremia, and it is a non-peptide selective antidiuretic hormone receptor antagonist. The drug can increase the concentration of sodium ions in the plasma, and help the excess water discharge from the urine. The drug could enhance the ability of the kidney to deal with water, and significantly reduce the weight and edema of patients while not accompanied by increased electrolyte excretion, without destroying the blood electrolyte balance. The drug can be used to treat hyponatremia caused by congestive heart failure, various edematous diseases, cirrhosis and antidiuretic hormone deficiency syndrome. The study found that, when the plasma sodium concentration decreased, in order to maintain the balance of sodium concentration inside and outside the cell, extracellular fluid will enter the cell, so the cells will swell. When the brain cells swell, it will lead to a variety of hyponatremia symptoms, including dizziness, weakness, headache, nausea, confusion, consciousness and convulsions. Severe hyponatremia can lead to coma and death. There is no corresponding study of tolvaptan tablets in patients with severe hyponatremia. | [Synthesis]
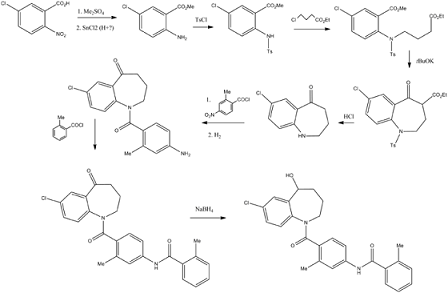
Fugure 1: Synthesis of Tolvaptan | [Precautions]
Tolvaptan(SAMSCA) should be initiated and re-initiated in patients only in a hospital where serum sodium can be monitored closely. Too rapid correction of hyponatremia (e.g., >12 mEq/L/24 hours) can cause osmotic demyelination resulting in dysarthria, mutism, dysphagia, lethargy, affective changes, spastic quadriparesis, seizures, coma and death. In susceptible patients, including those with severe malnutrition, alcoholism or advanced liver disease, slower rates of correction may be advisable.
Tolvaptan(SAMSCA) is contraindicated in the following conditions:
- Urgent need to raise serum sodium acutely
- Inability of the patient to sense or appropriately respond to thirst
- Hypovolemic hyponatremia
- Concomitant use of strong CYP 3A inhibitors
- Anuric patients
- Hypersensitivity (e.g. anaphylactic shock, rash generalized) to tolvaptan or its components
| [References]
https://www.samsca.com/dosing-and-administration
https://en.wikipedia.org/wiki/Tolvaptan |
|
|