| Identification | Back Directory | [Name]
L-3,4-DIHYDROXYPHENYLALANINE METHYL ESTER HYDROCHLORIDE | [CAS]
1421-65-4 | [Synonyms]
Nsc295453
Melevodopa
Levodopa Impurity 10
LevoMet Hydrochloride
Dopamine, methyl ester
methyldopa hydrochloride
Melevodopa Hydrochloride
H-TYR(3-HYDROXY)-OME HCL
METHYL L-DOPA HYDROCHLORIDE
H-PHE(3,4-DI-HYDROXY)-OME HCL
L-DOPA Me ester hydrochloride
L-DOPA Methyl Ester Hydrochloride
Levodopa methyl ester hydrochloride
-Methyl 2-amino-3-(3,4-dihydroxyphenyl)
L-3,4-Dihydroxyphenylalanine methyl ester
Methyl 3-Hydroxy-L-tyrosine Hydrochloride
3-Hydroxy-L-tyrosine Methyl Ester Hydrochloride
L-3,4-dihydroxyphenylalanine methyl*ester hydroch
L-3,4-DIHYDROXYPHENYLALANINE METHYLESTER HYDROCHLO
L-Tyrosine, 3-hydroxy-, methyl ester, hydrochloride
4-DIHYDROXYPHENYLALANINE METHYL ESTER HYDROCHLORIDE
3,4-DIHYDROXY PHENYLALANINE METHYL ESTER HYDROCHLORIDE
L-3,4-DIHYDROXYPHENYLALANINE METHYL ESTER HYDROCHLORIDE
3-hydroxy- L-Tyrosine methyl ester, hydrochloride (1:1)
Methyl L-3-(3,4-dihydroxyphenyl)alaninate hydrochloride
3,4-Dihydroxyphenyl-L-alanine methyl ester hydrochloride
L-3, 4-Dihydroxyphenylalanine methyleester hydrochloride
3,4-Dihydroxy-L-phenylalanine Methyl Ester Hydrochloride
3-(3,4-Dihydroxyphenyl)alanine Methyl Ester Hydrochloride
L-Tyrosine, 3-hydroxy-, methyl ester, hydrochloride (1:1)
L-3,4-Dihydroxyphenylalanine Methyl Ester Hydrochloride,>95%
(S)-2-Amino-3-(3,4-dihydroxyphenyl)propanoic acid hydrochloride
L-3,4-Dihydroxyphenylalanine methyl ester hydrochloride
[(2S)-3-(3,4-dihydroxyphenyl)-1-methoxy-1-oxopropan-2-yl]azanium
Levodopa methyl ester hydrochloride
L-DOPA Me ester hydrochloride
(S)-Methyl 2-aMino-3-(3,4-dihydroxyphenyl)propanoate hydrochloride
methyl (2S)-2-amino-3-(3,4-dihydroxyphenyl)propanoate hydrochloride
2-Amino-3-(3,4-dihydroxyphenyl)-propanoic acid methylester hydrochloride
L-3,4-Dihydroxyphenylalanine Methyl ester hydrochloride
TIANFUCHEM--1421-65-4--High purity L-3,4-DIHYDROXYPHENYLALANINE METHYL ESTER HYDROCHLORIDE in stock | [Molecular Formula]
C10H14ClNO4 | [MDL Number]
MFCD00038958 | [MOL File]
1421-65-4.mol | [Molecular Weight]
247.68 |
| Chemical Properties | Back Directory | [Melting point ]
174-175℃ | [alpha ]
+9.0° to +11.0° (20°C, 589nm) (C=2 in methanol) | [storage temp. ]
−20°C
| [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
solid
| [color ]
white
| [InChI]
InChI=1/C10H13NO4.ClH/c1-15-10(14)7(11)4-6-2-3-8(12)9(13)5-6;/h2-3,5,7,12-13H,4,11H2,1H3;1H/t7-;/s3 | [InChIKey]
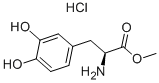
WFGNJLMSYIJWII-WMASNCOMNA-N | [SMILES]
C1(=CC=C(O)C(O)=C1)C[C@H](N)C(=O)OC.Cl |&1:9,r| |
| Hazard Information | Back Directory | [Description]
L-DOPA (Item No. 13248,1421-65-4) is a metabolic precursor of dopamine that is capable of crossing the blood brain barrier to act as a dopamine D1 receptor agonist. It is produced from L-tyrosine by trysosine hydroxylase. In the brain, L-DOPA is converted to dopamine by the enzyme aromatic L-amino acid decarboxylase. It is conventionally used to increase dopamine concentrations in the brain as a treatment for Parkinson’s disease and stroke recovery.1 L-DOPA methyl ester is a neutral derivative of L-DOPA formulated for increased solubility compared to the parent compound.2
| [Chemical Properties]
White Solid | [Uses]
Precursor to L-DOPA that crosses the blood-brain barrier; antiparkinsonian agent. | [Uses]
Selective Dopamine D1 receptor agonist. | [Biochem/physiol Actions]
L-3,4-Dihydroxyphenylalanine methyl ester(1421-65-4) is a precursor to?L-DOPA that crosses the blood-brain barrier. Antiparkinsonian agent L-DOPA methyl ester elicits antitumor functionality in leukemia and melanoma.
| [in vitro]
l-dopa methyl ester is a neutral derivative of l-dopa formulated for increased solubility compared to the parent compound. l-dopa is a metabolic precursor of dopamine that is capable of crossing the blood brain barrier to act as a dopamine d1 receptor agonist [1]. | [in vivo]
on intraperitoneal or subcutaneous administration to mice l-dopa methyl ester was found to be equivalent to l-dopa in reversing reserpine-induced akinesia and producing contraversive circling behaviour in rats. on oral administration the methyl ester was even more active. the administration of l-dopa or the methyl ester had equivalent changes of metabolite levels in striatal and mesolimbic dopamine, homovanillic acid, as well as 3,4-dihydroxyphenylacetic acid [2]. | [storage]
Store at -20°C | [References]
[1] berends, h. i.,nijlant, j.m.m.,movig, k.l.l., et al. the clinical use of drugs influencing neurotransmitters in the brain to promote motor recovery after stroke; a cochrane systematic review. european journal of physical and rehabilitation medicine 45(4), 621-630 (2009).
[2] cooper dr, marrel c, testa b, van de waterbeemd h, quinn n, jenner p, marsden cd. l-dopa methyl ester--a candidate for chronic systemic delivery of l-dopa in parkinson's disease. clin neuropharmacol. 1984;7(1):89-98.
[3] kleedorfer, b. ,lees, a.j. and stern, g.m. subcutaneous and sublingual levodopa methyl ester in parkinson’s disease. j.neurol.neurosurg.psychiatry 54(4), 373 (1991). |
|
|