| Identification | Back Directory | [Name]
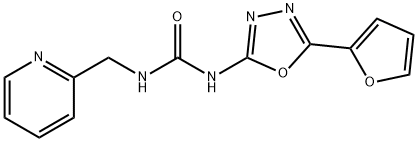
N-[5-(2-Furanyl)-1,3,4-oxadiazol-2-yl]-N′-(2-pyridinylmethyl)-urea | [CAS]
1414963-82-8 | [Synonyms]
NK 252;NK252
NK-252 >=98% (HPLC)
N-[5-(2-Furanyl)-1,3,4-oxadiazol-2-yl]-N′-(2-pyridinylmethyl)-urea
Urea, N-[5-(2-furanyl)-1,3,4-oxadiazol-2-yl]-N'-(2-pyridinylmethyl)- | [Molecular Formula]
C13H11N5O3 | [MDL Number]
MFCD28009512 | [MOL File]
1414963-82-8.mol | [Molecular Weight]
285.26 |
| Chemical Properties | Back Directory | [density ]
1.408±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
DMSO: soluble20mg/mL, clear | [form ]
powder | [pka]
11.50±0.70(Predicted) | [color ]
white to beige |
| Hazard Information | Back Directory | [Description]
NK 252 is a Nrf2 activator that interacts directly with the domain containing the Nrf2-binding site of Keap1. At 1.36 μM, it is reported to activate the NQO1-antioxidant response element in a luciferase reporter gene assay two-fold above background. NK 252 has been shown to downregulate the expression of fibrogenic genes, producing antifibrotic effects in a rat model of non-alcoholic steatohepatitis. | [Uses]
NK 252 is a Nrf2-activator. It exhibits a greater Nrf2-activating potential than OPZ. | [storage]
Store at +4°C | [References]
[1]. shimozono r, asaoka y, yoshizawa y, et al. nrf2 activators attenuate the progression of nonalcoholic steatohepatitis-related fibrosis in a dietary rat model. mol pharmacol. 2013 jul;84(1):62-70.
[2]. kiue a, sano t, naito a, et al. reversal by two dihydropyridine compounds of resistance to multiple anticancer agents in mouse p388 leukemia in vivo and in vitro. jpn j cancer res. 1990 oct;81(10):1057-64. |
|
| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Website: |
https://www.bocsci.com |
| Company Name: |
Musechem
|
| Tel: |
+1-800-259-7612 |
| Website: |
www.musechem.com |
| Company Name: |
Twochem Co.Ltd.
|
| Tel: |
021-58111628 15800915896 |
| Website: |
cn.twochem.com |
|