| Identification | Back Directory | [Name]
2'-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)amino)-7',8'-dihydro-6'H-spiro[cyclohexane-1,9'-pyrazino[1',2':1,5]pyrrolo[2,3-d]pyrimidin]-6'-one | [CAS]
1374743-00-6 | [Synonyms]
2'-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)amino)-7',8'-dihydro-6'H-spiro[cyclohexane-1,9'-pyrazino[1',2':1,5]pyrrolo[2,3-d]pyrimidin]-6'-one
2'-((5-(4-methylpiperazin-1-yl) yridine-2-yl)amino)-7',8'-dihydro-6'H-spiro[cyclohexane-1,9'-pyrazino[1',2':1,5]pyrrolo[2,3-d]pyrimidin]-6'-one
Spiro[cyclohexane-1,9'(6'H)-pyrazino[1',2':1,5]pyrrolo[2,3-d]pyrimidin]-6'-one, 7',8'-dihydro-2'-[[5-(4-methyl-1-piperazinyl)-2-pyridinyl]amino]- | [Molecular Formula]
C24H30N8O | [MDL Number]
MFCD30747890 | [MOL File]
1374743-00-6.mol | [Molecular Weight]
446.55 |
| Chemical Properties | Back Directory | [density ]
1.46±0.1 g/cm3(Predicted) | [solubility ]
H2O:15.0(Max Conc. mg/mL);33.59(Max Conc. mM) | [form ]
solid | [pka]
13.33±0.20(Predicted) | [color ]
White to yellow |
| Hazard Information | Back Directory | [Description]
Trilaciclib is a small molecule, competitive inhibitor of cyclin
dependent kinases 4 and 6 (CDK4/6), with potential antineoplastic and
chemoprotective activities. | [Characteristics]
Class: serine/threonine kinase
Treatment: myelopreservation (IV)
Elimination half-life = 36 h
| [Uses]
Trilaciclib is a CDK4/6 inhibitor with IC50s of 1 nM and 4 nM for CDK4 and CDK6, respectively. | [Brand name]
CoselaTM | [Mechanism of action]
Trilaciclib is inhibits cyclin-dependant kinase 4 (CDK4) at a
concentration of 1 nmol/L and cyclin-dependent kinase 5 (CDK5) at 4
nmol/L. Inhibition of CDK2, CDK5, and CDK7 is over 1000-fold less at
these concentrations and inhibition of CDK9 is 50-fold less. CDK4 and
CDK5 are expressed in hematopoietic stem cells and progenitor cells.
They are capable of phosphorylating and inactivating the retinoblastoma
protien; a tumor suppressor. When trilaciclib is given to patients with
retinoblastoma protein-null small cell lung cancer, it does not
interfere with the intended chemotherapy induced cytotoxicity of cancer
cells. Inhibition of CDK4 and CDK5 leads to a reversible pause in the
cell cycle in the G1 phase for approximately 16 hours. The temporary
cell cycle arrest prevents chemotherapy induced DNA damage in healthy
cells, reducing the activity of caspases 3 and 7, which reduces
apoptosis of healthy cells. Other studies have shown inhibitors of CDK4
and CDK6 enhance T-cell activation, upregulating major
histocompatibility complex (MHC) class I and II, and stabilize
programmed death-ligand 1 (PD-L1). Together these activities increase
T-cell activity, increase antigen presentation, and sensitize cells to
immune checkpoint inhibitors. | [Pharmacology]
Trilaciclib is indicated to reduce the incidence of chemotherapy induced myelosuppression in patients prior to receiving platinum and etoposide-containing or topotecan-containing
chemotherapy regimens for extensive-stage small cell lung cancer. It
has a short duration of action of approximately 16 hours, and a narrow
therapeutic index. Patients should be counselled regarding the risk of
injection site reactions, hypersensitivity, and interstitial lung
disease. | [Synthesis]
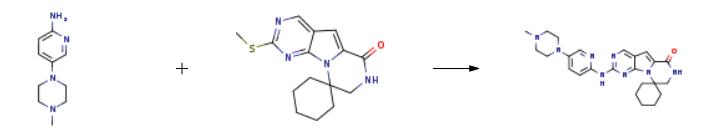
The synthesis of trilaciclib is as follows:
Intermediate 2 (21.1 g, 70 mmol) and 1-methyl-4-(6-aminopyridin-3-yl)piperazine (13.4 g, 70 mmol) were added to a 100 mL vacuum tube and reacted at 230° C. for 3 h.After the reaction, 300 mL of ethyl acetate was added, washed with water (120 mL) and saturated brine (120 mL) successively, dried with anhydrous sodium sulfate and filtered, the obtained solution was evaporated under reduced pressure to remove the solvent, and the obtained residue was recrystallized with ethanol, 27.5 g of Trilaciclib was obtained with a purity of 99% and a yield of 88%.
| [Drug interactions]
Trilaciclib is indicated to reduce the incidence of chemotherapy induced myelosuppression in patients prior to receiving platinum and etoposide-containing or topotecan-containing chemotherapy regimens for extensive-stage small cell lung cancer. | [storage]
Dry, dark and at 0 - 4℃ for short term (days to weeks) or -20℃ for long term (months to years). | [Mode of action]
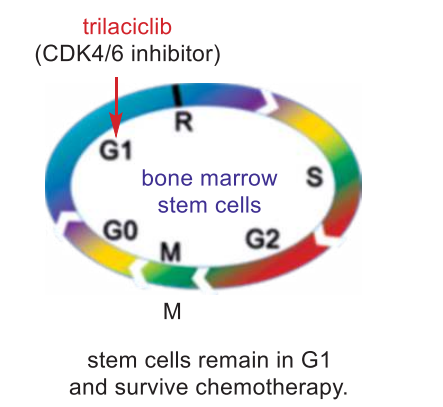
The G1 phase (where the cell is preparing to divide) to the S phase where DNA synthesis starts, and inhibition of CDK4/6 causes stem cells to temporarily stay at the G1 phase. In other words, the stem cells stop moving on to the S phase (DNA synthesis) or the G2 phase (growth), and certainly not to the M phase (mitosis). As a result, stem cells are protected from being damaged or even destroyed as chemotherapy only affects cells that are in other phases: the S, the G2, and the M phase. In the meantime, the tumor cells continue to transition to the S and M phases to divide, and they are killed by chemotherapy, causing the tumor to shrink. By preventing the stem cells from injury during chemotherapy, trilaciclib allows the patients to complete their course of treatment on time, and it avoids the expensive treatment of potential myelosuppressive adverse effects. So far, trilaciclib does not appear to reduce the efficacy of chemotherapy. In biochemical assays, trilaciclib reversibly inhibited CDK4/cyclin D1 and CDK6/cyclin D3 with an IC50 of 1 and 4 nM, respectively. It also displayed high selectivity against other CDK/cyclin complex family members and other protein kinases.
 |
|
| Company Name: |
ExSyn Corp
|
| Tel: |
+91-2240546474 +91-2240546474 |
| Website: |
www.exsyncorp.com |
|