| Identification | Back Directory | [Name]
1-Isopropyl-1H-pyrazole-5-boronic acid, pinacol ester | [CAS]
1282518-60-8 | [Synonyms]
1-Isopropylpyrazole-5-boronic Acid Pinacol Ester
1-Isopropyl-1H-pyrazole-5-boronic acid, pinacol ester
(1-Isopropyl-1H-Pyrazol-5-YL)Boronic Acid Pinacol Ester
1-Isopropyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)
1-(propan-2-yl)-5-(tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1-propan-2-yl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole
1-Isopropyl-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1-(1-Methylethyl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1H-Pyrazole, 1-(1-Methylethyl)-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-
3-hydroxy-2,3-dimethylbutan-2-yl hydrogen (1-isopropyl-1H-pyrazol-5-yl)boronate | [Molecular Formula]
C12H21BN2O2 | [MDL Number]
MFCD17167306 | [MOL File]
1282518-60-8.mol | [Molecular Weight]
236.12 |
| Chemical Properties | Back Directory | [Boiling point ]
327.3±15.0 °C(Predicted) | [density ]
1.03±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [form ]
solid | [pka]
1.98±0.10(Predicted) | [color ]
White to off-white |
| Hazard Information | Back Directory | [Synthesis]
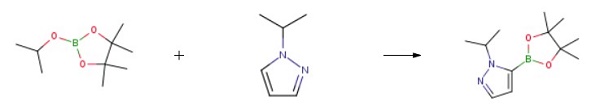
1-Isopropyl-1H-pyrazole-5-boronic acid, pinacol ester could be synthesized through the reaction of 1-isopropyl-1H-pyrazole with 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane. Tetrahydrofuran could used as an organic solvent. The crude product added n-hexane, heated to 40°C to dissolve the system, cooled to -20°C, stirred and crystallized, and filtered. Vacuum drying to obtain the target compound 1-Isopropyl-1H-pyrazole-5-boronic acid, pinacol ester, with a yield of 77% and a purity of 99.3%.
 |
|
|