| Identification | Back Directory | [Name]
4-{[6-(Acryloyloxy)Hexyl]Oxy} Phenyl Hydrogen Trans-Cyclohexane-1,4-Dicarboxylate | [CAS]
1173478-72-2 | [Synonyms]
trans-4-[4-(6-Acryloxyhexyloxy)phenoxycarbonyl]cyclohexanecarboxylic acid
4-{[6-(Acryloyloxy)Hexyl]Oxy} Phenyl Hydrogen Trans-Cyclohexane-1,4-Dicarboxylate
trans-4-((4-((6-(acryloyloxy)hexyl)oxy)phenoxy)carbonyl)cyclohexanecarboxylic acid
1,4-Cyclohexanedicarboxylic acid, 1-[4-[[6-[(1-oxo-2-propen-1-yl)oxy]hexyl]oxy]phenyl] ester, trans- | [Molecular Formula]
C23H30O7 | [MOL File]
1173478-72-2.mol | [Molecular Weight]
418.48 |
| Hazard Information | Back Directory | [Chemical Properties]
4-{[6-(Acryloyloxy)Hexyl]Oxy} Phenyl Hydrogen Trans-Cyclohexane-1,4-Dicarboxylate is an intermediate compound with the appearance of a white powder. Store at low temperature and dry. | [Uses]
4-{[6-(Acryloyloxy)Hexyl]Oxy} Phenyl Hydrogen Trans-Cyclohexane-1,4-Dicarboxylate is a liquid crystal monomer material that can be used to produce LCD products. | [Preparation]
The preparation of 4-{[6-(Acryloyloxy)Hexyl]Oxy} Phenyl Hydrogen Trans-Cyclohexane-1,4-Dicarboxylate is as follows:0.64 g (5.22 mmol) of 4-(dimethylamino)pyridine and 13.80 g (52.21 mmol) of 4-(6-acryloyloxyhex-1-yloxy)phenol (manufactured by DKSH) were added to the obtained reaction solution, and the reactor was again immersed in a water bath to adjust the temperature of the reaction solution to 15° C. 6.34 g (62.65 mmol) of triethylamine was dropped over 10 minutes in the solution while maintaining the internal temperature of the reaction solution at 20° C. to 30° C., and after the dropwise addition, the solution was stirred at 25° C. for an additional 2 hours. After completion of the reaction, 1000 ml of distilled water and 100 ml of saturated saline solution were added to the reaction solution, followed by extraction twice with 400 ml of ethyl acetate. The organic layer was collected, and dried with anhydrous sodium sulfate, and the sodium sulfate was filtered off. After the solvent was evaporated from the filtrate using a rotary evaporator, the obtained residue was purified by silica gel column chromatography (THF:toluene=1:9 (volume ratio)) to obtain 14.11 g of the compound M as a white solid. The yield was 65 mol %. 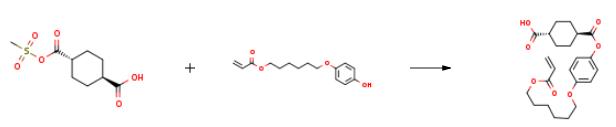
|
|
|