| Identification | Back Directory | [Name]
Erythropoietin | [CAS]
11096-26-7 | [Synonyms]
EPO
ESF
hEPO
Aids000135
Aids-000135
INN=Epoetin
ERYTHROPOIETIN
EPO 11096-26-7
ERYTHROPOIETIN RAT
Erythropietin human
Erythropoietin (100 ug)
Erythropoietin USP/EP/BP
Erythropoietin human,EPO
Erythropoietin from rat
Human EPO Protein, Fc Tag
Erythropoietin from mouse
Mouse Anti Human Erythropoietin
Erythropoietin,from human urine
ERYTHROPOIETIN HUMAN, RECOMBINANT 97+%
Erythromycin Human,recombinant,CHO Cell
Mouse Anti Human Erythropoietin clone NYRhEPO
erythropoietin from human urine*approx 100 units
RECOMBINANT HUMAN ERYTHROPOIETIN INJECTION (RH-EPO)
ERYTHROPOIETIN FROM HUMAN URINE*APPROX 5 00 UNITS PE
ERYTHROPOIETIN FROM HUMAN URINE*APPROX 100 UNITS PER
ERYTHROPOIETIN HUMAN, RECOMB. FROM CHO- CELLS, PGE W. 10 U
ANTI-ERYTHROPOIETIN(N-TERMINAL) antibody produced in rabbit
ANTI-ERYTHROPOIETIN(C-TERMINAL) antibody produced in rabbit | [EINECS(EC#)]
234-317-2 | [Molecular Formula]
NULL | [MDL Number]
MFCD03457594 | [MOL File]
11096-26-7.mol | [Molecular Weight]
461.001 |
| Chemical Properties | Back Directory | [Description]
Recombinant erythropoietin (EPO) is currently indicated for use only in anemia associated with renal transplant or end-stage renal disease. Widened indications for use in other forms of anemia, accompanied by price reductions as a result of keen competition, have been projected for EPO in the future. | [storage temp. ]
−20°C
| [form ]
lyophilized powder
| [color ]
white | [Merck ]
13,3722 | [InChI]
InChI=1S/C22H22ClN6O.K/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22;/h4-7,9-12,30H,2-3,8,13-14H2,1H3;/q-1;+1 | [InChIKey]
OXCMYAYHXIHQOA-UHFFFAOYSA-N |
| Questions And Answer | Back Directory | [Discovery]
This is the primary regulator of erythropoiesis. It is produced predominantly in the fetal liver and the adult kidney to
promote the survival, proliferation, and differentiation of
erythroid progenitor cells. Other nonhematopoietic roles
include neuro-, cardio-, and reno-protection as well as
wound healing. The relationship between anemia and hypoxia/atmospheric pressure was perceived around the 16th century,
predicted experimentally by Carnot and Deflandre. The
tentative name erythropoietin (EPO) was proposed independently by Komiya and by Bonsdorff and Jalavisto. Reissmann and Erslev confirmed the humoral activity
in the blood. Jacobson and colleagues found that the kidney primarily produced EPO. The human EPO protein
was finally purified directly from approximately 2550 L
of human urine of patients with aplastic anemia. The
subsequent molecular cloning of cDNA and the genomic
DNA of human EPO was accomplished concurrently by
two research groups in 1985. | [Structure]
Mature EPOs are heavily glycosylated to
reach 30%–40% (w/w) of the whole molecule. The terminal sialic acids provide stability in the circulation, and
are essential for in vivo activity. The tertiary structure
displays four-α-helical bundles shared among typical
cytokines. The mature human EPO consists of 165 aa residues
after posttranslational cleavage of an Arg166 at the
C-terminus. Mr. of human EPO polypeptide backbone is 18.2 kDa,
34 kDa on SDS-PAGE (glycosylated native form), or more
than 40 kDa (fully glycosylated recombinant EPO
expressed by CHO cells). The isoelectric point (pH 3–5)
varies depending on glycosylation. The level of biological
activity is reasonable against short-term heat treatment,
6M urea, 6M GuHCl, or ordinary surfactants, when renaturation is performed properly.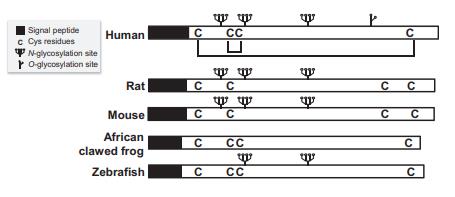 | [Gene, mRNA, and precursor]
The human EPO gene is located in the q11 to q22
region of chromosome 7 [NC_000007. (100,720,
800.100723700)]. Human EPO mRNA
encodes a 193-aa polypeptide. Heterogeneity in the size
of EPO mRNA has been reported; for example, EPO
mRNA of larger size is found in the brain. The production sites vary depending on either species
or ontogenic stages. In mammals, the predominant sites of
EPO production shift from the fetal liver to the adult kidney along with the transition of hematopoietic tissues. In
mice, hepatocytes in the fetus and adult renal cells in the
peritubular interstitium produce EPO. In nonmammalian vertebrates, EPO is generated predominantly in the
lung and the liver of the African clawed toad. In teleost fish, the heart is one of the major production sites of EPO.
EPO for murine primitive erythropoiesis is produced in
neuroepithelial and neural crest cells. | [Synthesis and release]
Human EPO regulates erythropoiesis in a hypoxiainducible manner. Levels of HIF-1α in EPO-producing cellsincrease exponentially as O2 concentration
declines because ubiquitination and proteasomal degradation of HIF-1αdecrease. Then EPO expression is directly
upregulated by the transcriptional activation via interaction of a 30 enhancer complex with the 50 promoter. The number of EPO-producing cells
in the human kidney correlates positively with circulating
levels of EPO, but the range of EPO expression per cell has
not been determined. Ninety percent of the circulating
EPO originates from the kidneys and the rest from various
organs, including the liver, brain, spleen, lung, and testis. | [Receptors]
The EPO receptor (EPOR) is a glycoprotein that belongs
to the type I superfamily of single-transmembrane cytokine receptors. A soluble form of EPOR lacking a
transmembrane region generated by the alternative splicing of EPOR mRNA is found in human blood. The tertiary
structure of human EPO and the homodimerized EPOR
complex has been determined. Intracellular EPO-EPOR signaling is triggered by the
binding of EPO, and EPORs homodimerize to activate
a cascade of JAK2 and STAT3/5, PI3K, and/or RAS/
MAPK. | [Agonists and Antagonists]
Small mimetic peptides such as EMP111 and their
derivatives, agonistic antibodies to EPOR that mimic
the conformation of EPO-EPOR binding (Ab12.6, also
known as ABT007), and EPO fused with hybrid immunoglobulin Fc (EPO-hyFc), have been reported. Other than specific antibodies to EPO or EPOR, soluble
EPOR inhibits the EPO/EPOR-dependent cell proliferation in glioma cells. | [Biological functions]
As a primary target of EPO for erythropoiesis, EPOR is
expressed in the organs of hematopoiesis, including the
fetal liver as well as the adult bone marrow and spleen.
EPO-EPOR signaling regulates the proliferation/differentiation and survival of the erythroid progenitors,
providing an important stage-specific function of erythroid differentiation. The numbers of EPOR expressed
in various cells are relatively low and range between
100 and 3000 per cell with the binding affinity (ED50) to
EPO ranging from 0.1–3 nM. A wide distribution of EPOR
expression is confirmed on renal cells, endothelial cells,
cardiomyocytes, the brain, and peripheral nervous system where EPO may exert pleiotropic or antiapoptotic
effects. | [Clinical implications]
EPO maintains the number of circulating red blood
cells, that is, hemoglobin levels, by stimulating the proliferation and differentiation of erythrocyte progenitors in
the bone marrow. Therefore, EPO is mainly administered
as hormonal replacement therapy to patients with renal
failure who have experienced the compromised production of EPO. Additionally, the nonhemopoietic role of
erythropoietin has been evaluated in clinical trials,
including the reduction of hyperglycemia and the retardation of proliferative retinopathy in diabetic patients. | [Use for diagnosis and treatment]
Recombinant human EPO has been among themost successful therapeutic biologics. Originally, epoetin-α and -β
produced by CHO cells were developed and launched
for the treatment of chronic renal anemia to achieve optimal
hemoglobin levels and improve the QOL. Its application
has extended to cancer-related anemia involving chemotherapy/radiation, inflammatory bowel disease
(Crohn’s disease and ulcer colitis), and others. The circulating levels of human EPO have been determined by RIA or
ELISA for the diagnosis of different types of anemia. The
second generation of erythropoiesis-stimulating agents
(ESAs), such as a long-acting analog darbepoetin with
two additional glycans andits various generics,is following
the first generation. Doping with EPO has become a serious
issue in athletics competitions. |
| Hazard Information | Back Directory | [Originator]
Amgen (USA) | [Uses]
Erythropoietin can be used in biological study of its attenuation of cardiac dysfunction in rats by inhibiting endoplasmic reticulum stress-induced diabetic cardiomyopathy. | [Brand name]
Eprex | [General Description]
EPO has been cloned from various species including human, murine, canine, and others. The mature proteins from the various species are highly conserved and exhibit greater than 80% amino acid sequence identity. EPO contains three N-linked glycosylation sites. The glycosylation of erythropoietin is required for the biological activities of erythropoietin in vivo. | [Biochem/physiol Actions]
Erythropoietin (EPO), produced primarily by the kidney, is the primary regulatory factor of erythropoiesis. It promotes the proliferation, differentiation, and survival of the erythroid progenitors. Erythropoietin stimulates erythropoiesis by inducing growth and differentiation of burst forming units and colony forming units into mature red blood cells. EPO produced by kidney cells is increased in response to hypoxia or anemia. The biological effects of erythropoietin are mediated by the erythropoietin receptor, which binds EPO with high affinity and is a potent EPO antagonist. |
|
|