| Identification | Back Directory | [Name]
N-BOC-1-AMINO-CYCLOPROPANEMETHANOL | [CAS]
107017-73-2 | [Synonyms]
SKL325
Boc-ACPC-ol
Boc-1-Aminocyclopropylmethanol
Boc-1-aminocyclopropane methanol
1-(Boc-amino)cyclopropylmethanol
N-Boc-1-AMinocyclopropylMethanol
N-BOC-1-AMINO-CYCLOPROPANEMETHANOL
[1-(tert-ButoxycarbonylaMino)cyclopropyl]Methanol
tert-Butyl [1-(Hydroxymethyl)cyclopropyl]carbamate
tert-butyl 2-(1-aMinocyclopropyl)-2-hydroxyacetate
(1-HydroxyMethyl-cyclopropyl)-carbaMic acid tert-b
tert-butyl N-[1-(hydroxymethyl)cyclopropyl]carbamate
[1-(tert-Butoxycarbonylamino)cyclopropyl]methanol >
N-BOC-1-AMINO-CYCLOPROPANEMETHANOL ISO 9001:2015 REACH
[1-(tert-Butoxycarbonylamino)cyclopropyl]methanol
TERT-BUTYL 1-(HYDROXYMETHYL)CYCLOPROPYLCARBAMATE(WX640242)
2-Methyl-2-propanyl [1-(hydroxyMethyl)cyclopropyl]carbaMate
[1-(Hydroxymethyl)cyclopropyl]carbamicacid tert-butyl ester
1-(Boc-amino)cyclopropylmethanol
N-Boc-1-amino-cyclopropanemethanol
Carbamic acid, N-[1-(hydroxymethyl)cyclopropyl]-, 1,1-dimethylethyl ester
Carbamicacid, [1-(hydroxymethyl)cyclopropyl]-, 1,1-dimethylethyl ester (9CI) | [Molecular Formula]
C10H19NO3 | [MDL Number]
MFCD09749954 | [MOL File]
107017-73-2.mol | [Molecular Weight]
187.24 |
| Chemical Properties | Back Directory | [Melting point ]
81.0 to 85.0 °C | [Boiling point ]
294.5±9.0 °C(Predicted) | [density ]
1.11±0.1 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
powder to crystal | [pka]
12.24±0.20(Predicted) | [color ]
White to Orange to Green | [InChI]
InChI=1S/C9H17NO3/c1-8(2,3)13-7(12)10-9(6-11)4-5-9/h11H,4-6H2,1-3H3,(H,10,12) | [InChIKey]
HFMAZNJKNNRONT-UHFFFAOYSA-N | [SMILES]
C(OC(C)(C)C)(=O)NC1(CO)CC1 |
| Hazard Information | Back Directory | [Mechanism of action]
User reports indicate that ephylone produces a mixture of classic stimulant and entactogenic effects resembling those of MDMA, methylone and cocaine. Typical effects include stimulation, disinhibition, increased libido, compulsive redosing, and euphoria. Unlike similar substances, however, ephylone is reported to be very long lasting when taken in larger doses. The significance of this is not known, although it may indicate that it has a different toxicity profile compared to other stimulants.
Ephylone is sold online as a research chemical alongside other synthetic cathinones like ethylone and dibutylone. Due to the lack of research, it is highly advised to use harm reduction practices if using this substance. | [Uses]
Tert-Butyl (1-(hydroxymethyl)cyclopropyl)carbamate could be converted into spirocyclopropanated analogues 14-CP and 14-CT of the insecticide Thiacloprid [1]. It can also be transformate of into spirocyclopropanated analogues of Imidacloprid.
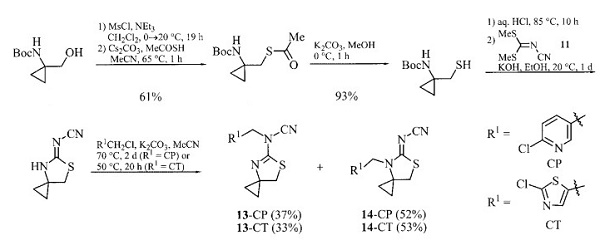 | [Preparation]
Tert-Butyl (1-(hydroxymethyl)cyclopropyl)carbamate can be synthesized in three steps (40 % overall yield) from N, Ndibenzyl-2-benzyloxyacetamide starting with its reductive cyclopropanation (the de Meijere variant of the so-called Kulinkovich reaction). It can also be prepared in 56 % overall yield by monohydrolysis of commercially available diethyl cyclopropane-1,1-dicarboxylate followed by Curtius degradation of the carboxylic acid residue and subsequent reduction of the ester moiety[1]. | [References]
[1] Brackmann F, et al. Synthesis of Spirocyclopropanated Analogues of Imidacloprid and Thiacloprid. European Journal of Organic Chemistry, 2005; 2005: 600-609.
[2] Brackmann F, et al. Synthesis of Spirocyclopropanated Analogues of Iprodione. European Journal of Organic Chemistry, 2005; 2005: 2250-2258. |
|
|