| Identification | Back Directory | [Name]
LX-4211 | [CAS]
1018899-04-1 | [Synonyms]
LX421
LX-4211
LP 802034
EOS-61381
Sotagliflozin
LX4211/LX-4211
LX-4211, >=98%
LX4211 - L10525
LX-4211 (sotagliflozin)
Zabofloxacin Hydrochloride
LX4211, 98%, a potent dual SGLT2/1 inhibitor
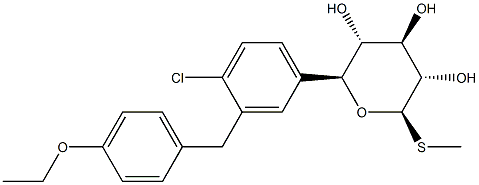
(5S)-Methyl 5-C-[4-chloro-3-[(4-ethoxyphenyl)Methyl]phenyl]-1-thio-beta-L-xylopyranoside
(2S,3R,4R,5S,6R)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-methylsulfanyloxane-3,4,5-triol
(2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(Methylthio)tetrahydro-2H-pyran-3,4,5-triol
(5S)-Methyl 5-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-1-thio-beta-L-xylopyranoside Sotagliflozin LP 802034 | [Molecular Formula]
C21H25ClO5S | [MDL Number]
MFCD22493506 | [MOL File]
1018899-04-1.mol | [Molecular Weight]
424.938 |
| Chemical Properties | Back Directory | [Boiling point ]
607.9±55.0 °C(Predicted) | [density ]
1.37±0.1 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO:84.0(Max Conc. mg/mL);197.67(Max Conc. mM)
Ethanol:17.0(Max Conc. mg/mL);40.01(Max Conc. mM) | [form ]
Powder | [pka]
12.87±0.70(Predicted) | [color ]
White to off-white | [InChIKey]
QKDRXGFQVGOQKS-WFCPIQDQNA-N | [SMILES]
[C@@H]1(C2=CC=C(Cl)C(CC3=CC=C(OCC)C=C3)=C2)O[C@H](SC)[C@@H](O)[C@H](O)[C@H]1O |&1:0,19,22,24,26,r| |
| Hazard Information | Back Directory | [Uses]
Sotagliflozin is used for the treatment of patients with type 1 diabetes mellitus. | [Clinical Use]
Two of the current SGLT-2 inhibitors (canagliflozin and LX-4211) have effects on SGLT-1. LX-4211 is a dual SGLT1/2 inhibitor and higher doses of canagliflozin transiently inhibit SGLT-1 in the gut due to high canagliflozin concentrations within the intestinal lumen during the period of drug absorption. | [Synthesis]
Sotagliflozin is a dual SGLT-1/SGLT-2 inhibitor which is currently under development by Lexicon Pharmaceuticals (phase III). It follows a similar strategy to ertugliflozin, i.e. aryl addition on an acyclic precursor. The synthesis starts from L-xylose 53, and the aryl moiety (same aryl moiety as for dapagliflozin) is introduced on an amide derivative (morpholine amide 54) via Grignard addition. A subsequent transformation leads to the sotagliflozin (Scheme 10). The overall yield of the synthesis is around 30%.

Sotagliflozin synthesis
|
|
|